All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
AACR 2017 | Novel therapeutic opportunities for Germinal Center B-Cell Lymphomas
Bookmark this article
On April 5th 2017, during this year’s American Association for Cancer Research (AACR) annual meeting, Laura Pasqualucci, from Columbia University, New York, gave a presentation on the topic of “Novel therapeutic opportunities for germinal center B-cell lymphomas”. This presentation was part of the session titled “Novel Targets in Hematologic Malignancies”.
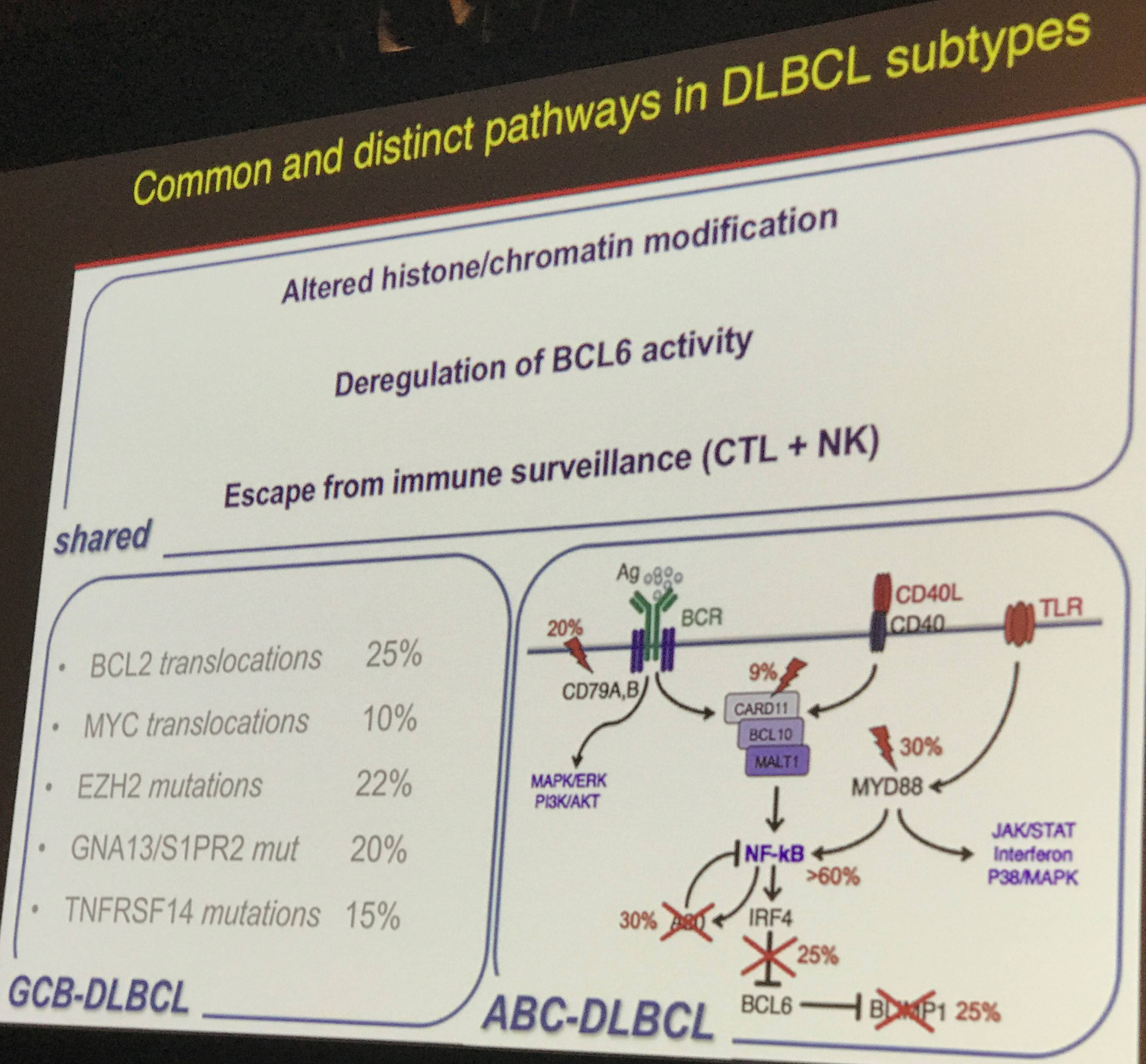
Professor Pasqualucci began her talk by explaining that DLBCL has “remarkable heterogeneity in morphologic, molecular, and clinical features”, and outlined the recognized phenotypic subgroups of DLBCL as being GCB-DLBCL, ABC-DLBCL, and PMBCL. The GCB- and ABC-DLBCL subtypes share common aberrant molecular pathways such as deregulation of BCL6, however, they have major differences in other pathways which were highlighted on this slide:
Epigenetics and transcriptional modification also play different roles in different lymphoma subtypes. Below is an overview of some of the known changes to epigenetic and transcriptional proteins; of note is that 25% of DLBCL cases have mutations in CREBBP or EP300, and 30% have mutations in MLL2, part of the COMPASS complex which de-condenses chromatin.
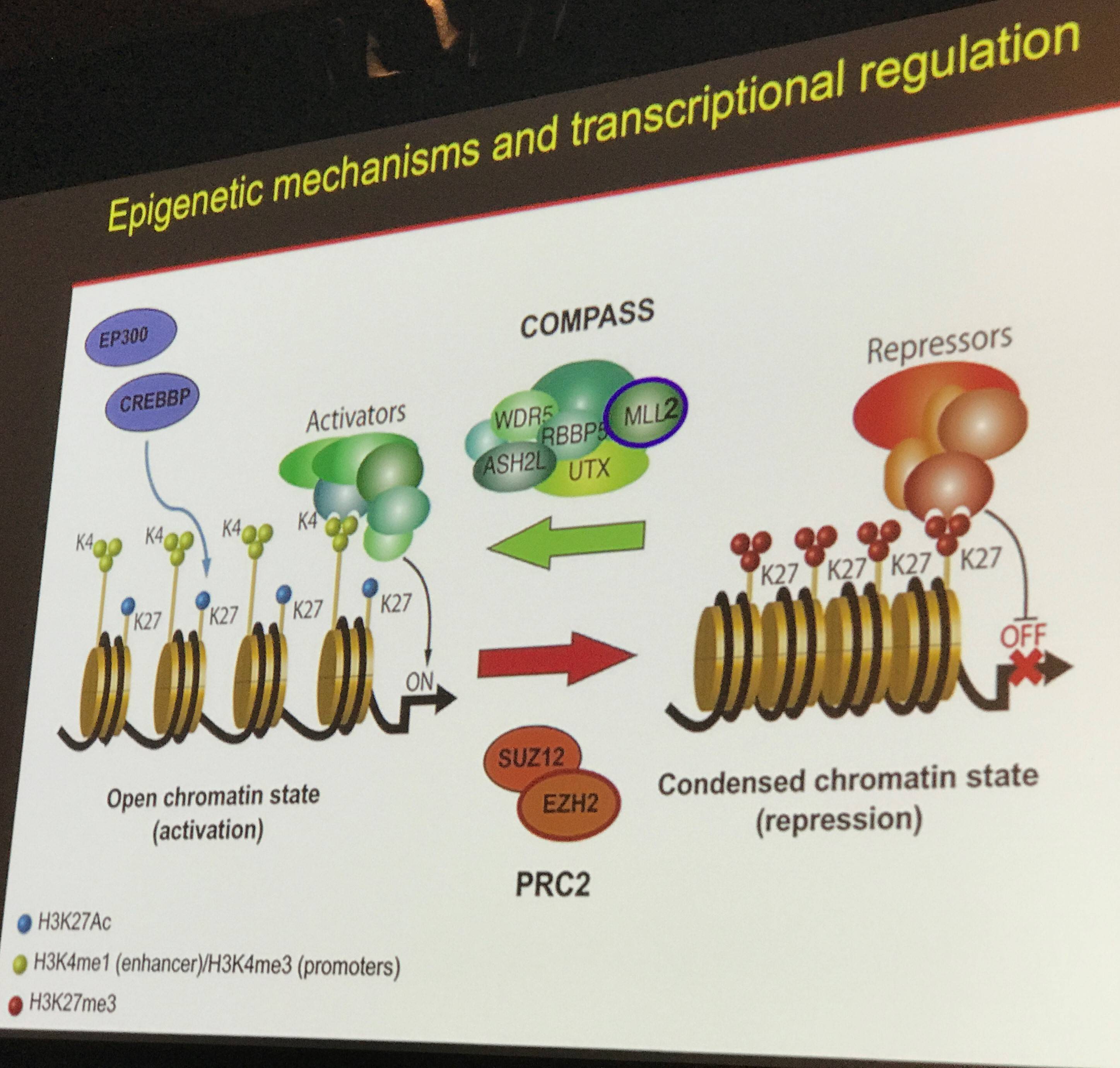
The mutations in MLL2 and CREBBP have been shown to result in protein truncation at the C-terminus, which is where the catalytic domain of these proteins reside, thereby resulting in impaired protein function. Data was also shown that suggested that these mutations are monoallelic, potentially meaning that they have a haploinsufficient tumor suppressor function.
In 2015, using mouse models, it was found that MLL2 loss resulted in perturbed formation of germinal centers, and was found to be able to cooperate with BCL2 deregulation in FL and DLBCL lymphomas, as shown by BCL2 overexpressing mice who had MLL2 genetic ablation.
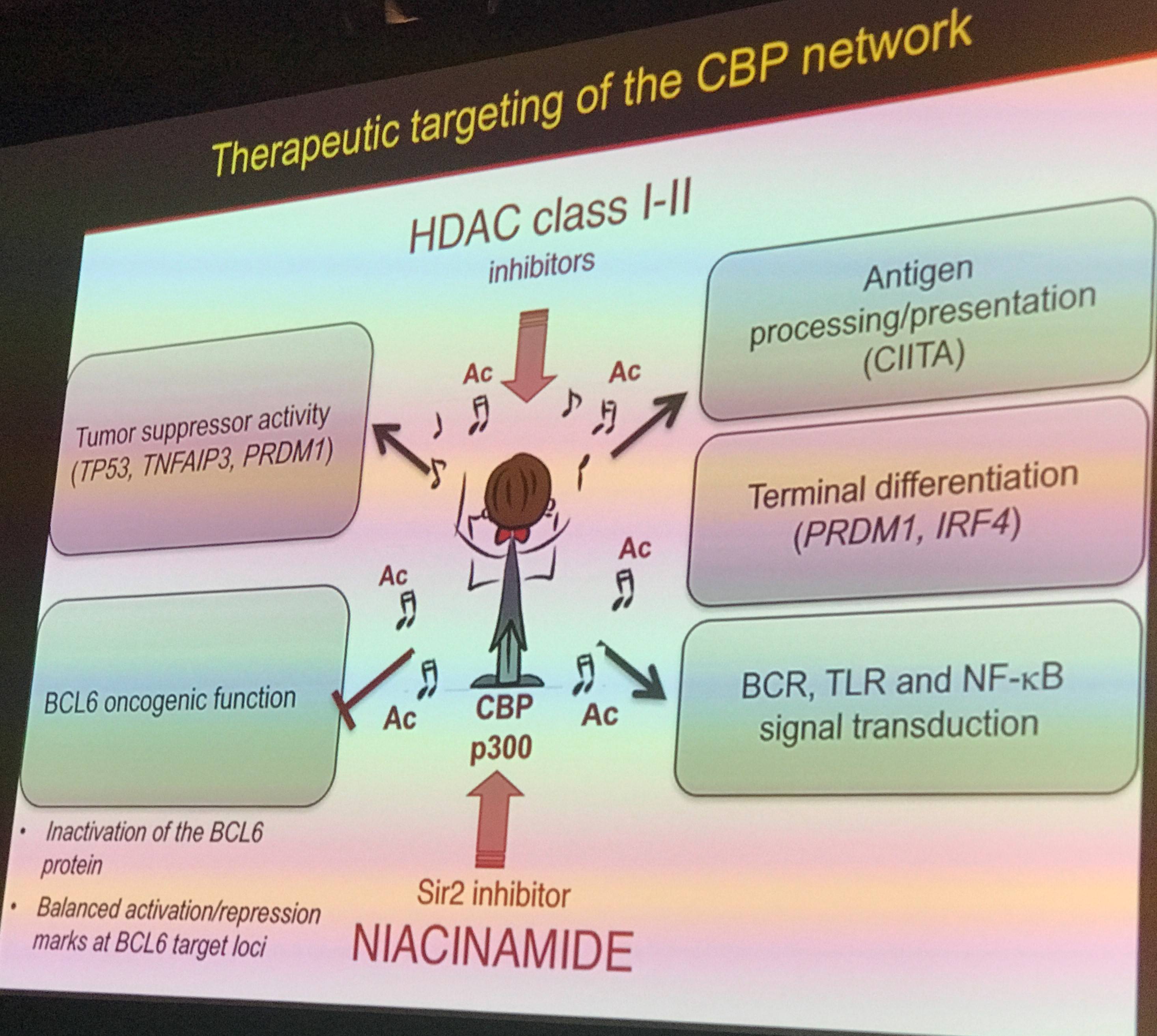
Professor Pasqualucci next spoke about the role of inactivating CBP (CREB-binding protein)/p300 in DLBCL and FL. She stated that inactivation of CBP/p300 relieves the inactivation of BCL6, allowing it to inhibit p53, resulting in lower p53 activation. As p53 is a tumor suppressor, its reduced activation may contribute to oncogenesis.
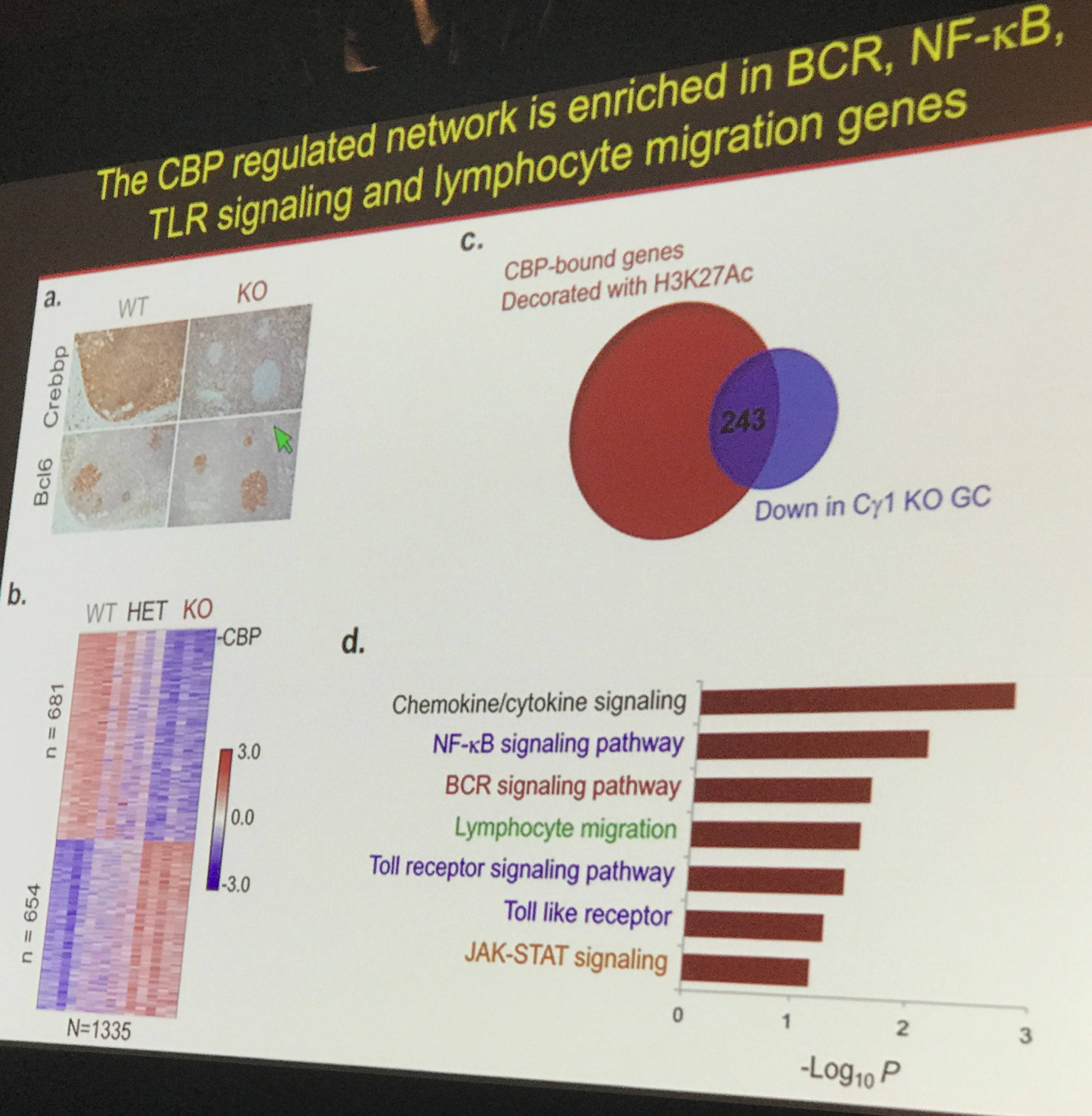
Data presented based on a variety of transcriptional analyses showed that CBP regulates a network of proteins involved with BCR, NFkB, and Toll-like receptor signaling, in addition to signaling involved with lymphocyte motility.
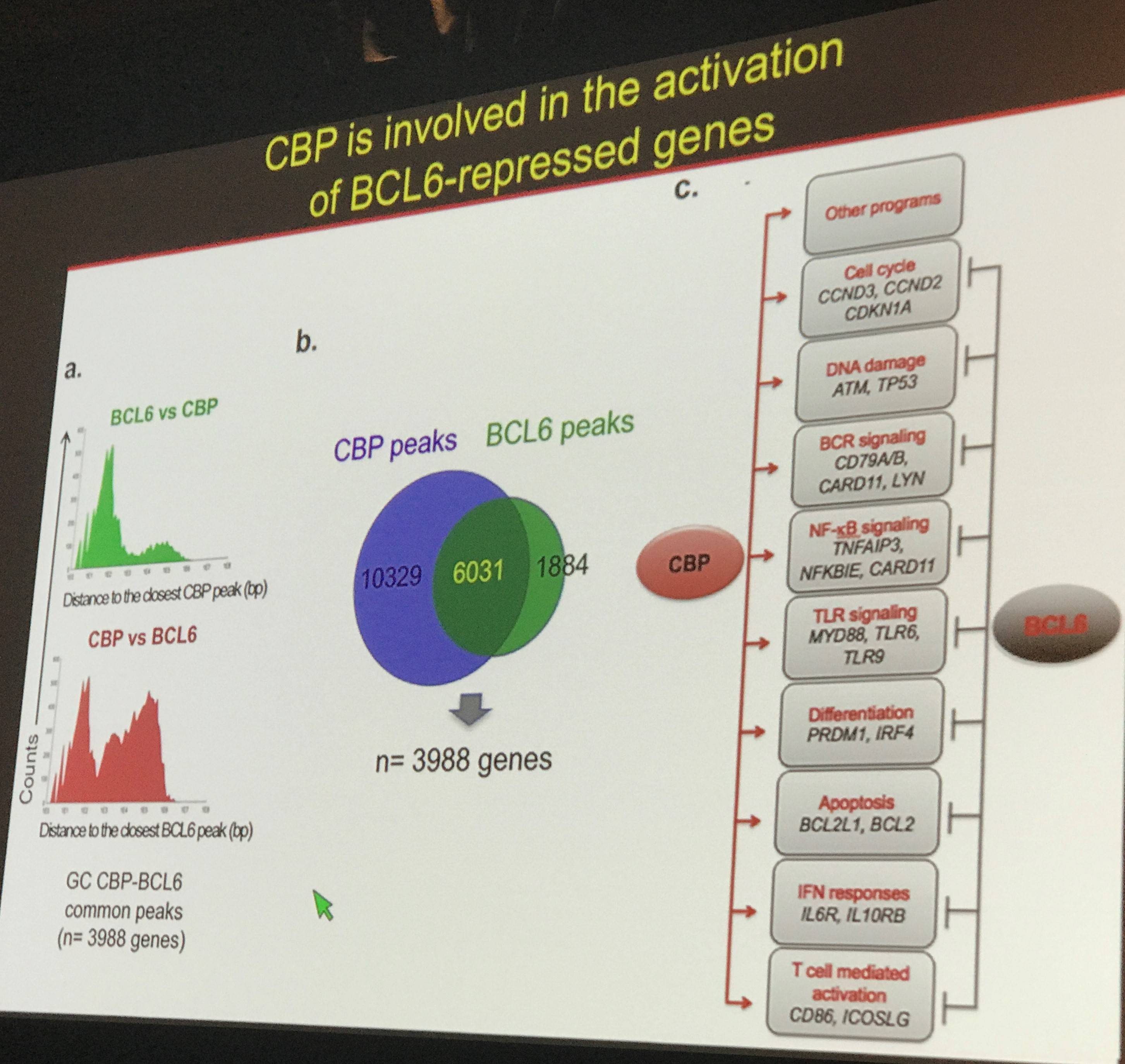
Professor Pasqualucci next went on to detail data that showed that CBP is involved with activating targets which are typically repressed by BCL6. The BCL6-repressed targets function in a variety of cellular processes including apoptosis, differentiation, and cell cycle regulation. Furthermore, it was shown that loss of a single allele of CBP resulted in B-cell lymphoma development via BCL2 (below).
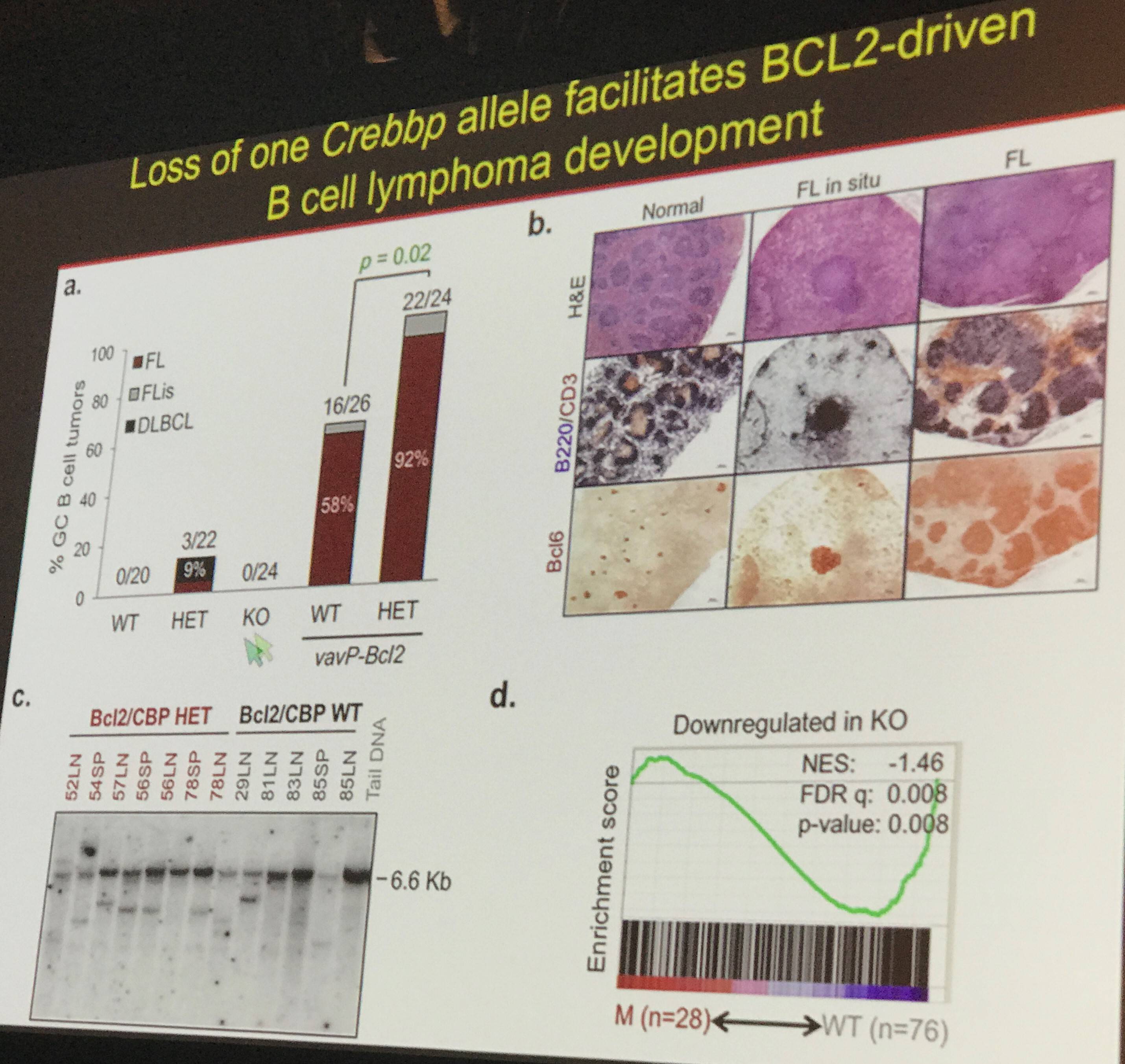
In summary, Professor Pasqualucci summarized that the role of CBP/p300 is to orchestrate key cellular processes through interaction with key transcription factors. However, when the activation of CBP/p300 is limited in germinal center cells, many downstream pathways are altered, resulting in aberrant cell behavior and, potentially, lymphomagenesis. In the future, targeting these pathways may help bring about new drugs to treat lymphomas where these mutations have occurred.
- Pasqualucci, L. Novel therapeutic opportunities for germinal center B-cell lymphomas [Presentation]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; 2017. Session nr [RADT13].

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








