All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
AACR 2017 | Polyfunctional anti-CD19 CAR T-cells associated with clinical activity in patients with advanced Non-Hodgkin Lymphoma
Bookmark this article
On Monday 3rd April at AACR 2017, during the “MS.CL10.01 - Clinical Biomarkers” session, John Rossi from Kite Pharma, Santa Monica, CA, gave a talk titled: “Polyfunctional anti-CD19 CAR T cells determined by single-cell multiplex proteomics associated with clinical activity in patients with advanced non-Hodgkin’s lymphoma.”
Rossi began the talk by providing some background on CD19 specific Chimeric Antigen Receptor (CAR) T-cell therapy:
- Anti-CD19 CAR T-cells have demonstrated clinical activity in advanced NHL (Kochenderfer Blood 2010 and JCO 2017)
- So far, the best correlate of objective response is expansion of CAR T-cells within 4 weeks post-infusion (Kochenderfer JCO 2017 and Locke AACR 2017)
- Pro-inflammatory, immune modulatory, immune effector, and chemokine signaling immune programs are initiated by anti-CD19 CAR T-cells (Rossi SITC 2016)
- No clear associations with clinical outcomes have been observed when evaluating bulk product
The talk then discussed how CAR T-cell polyfunctionality is quantified:
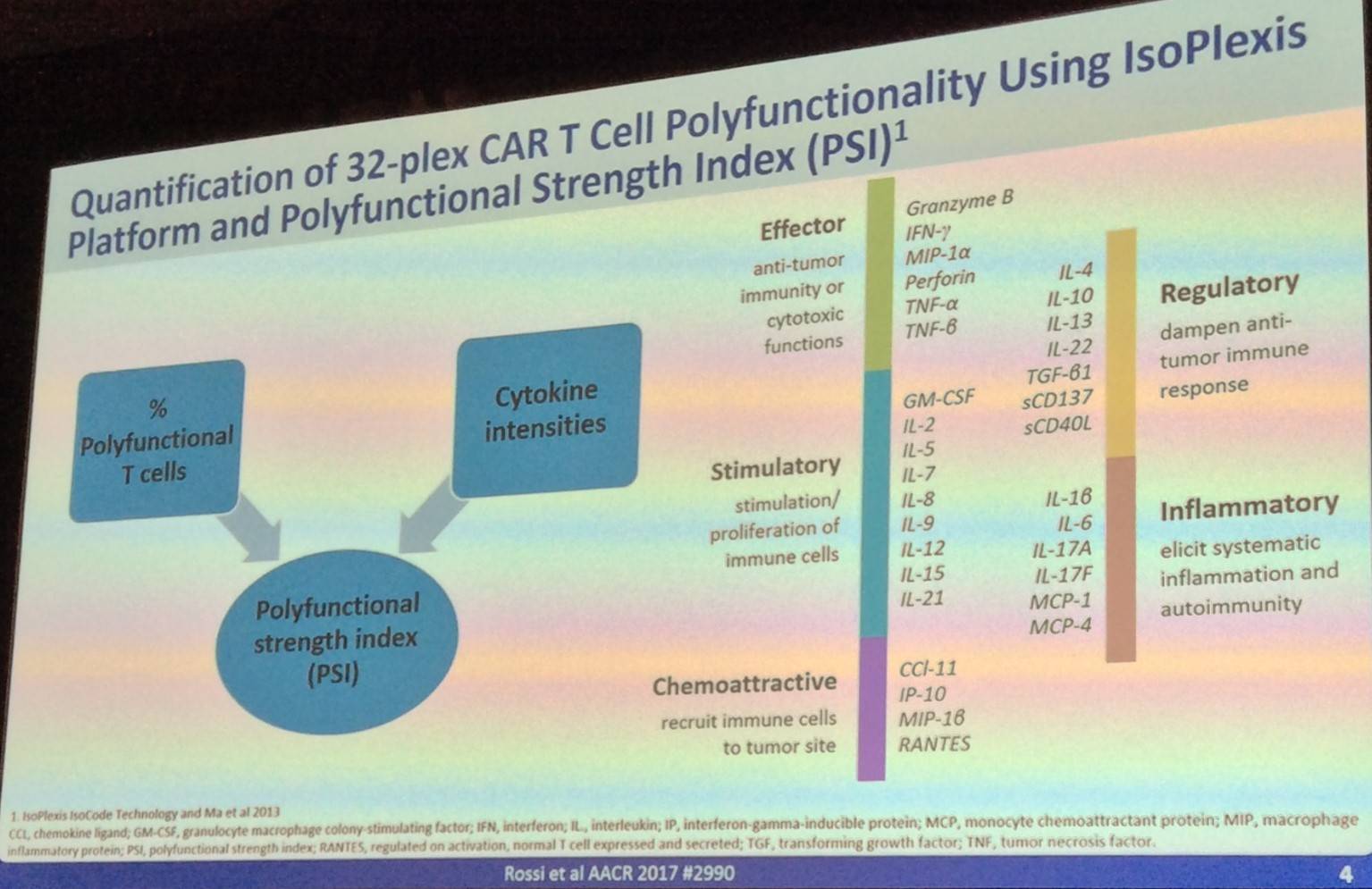
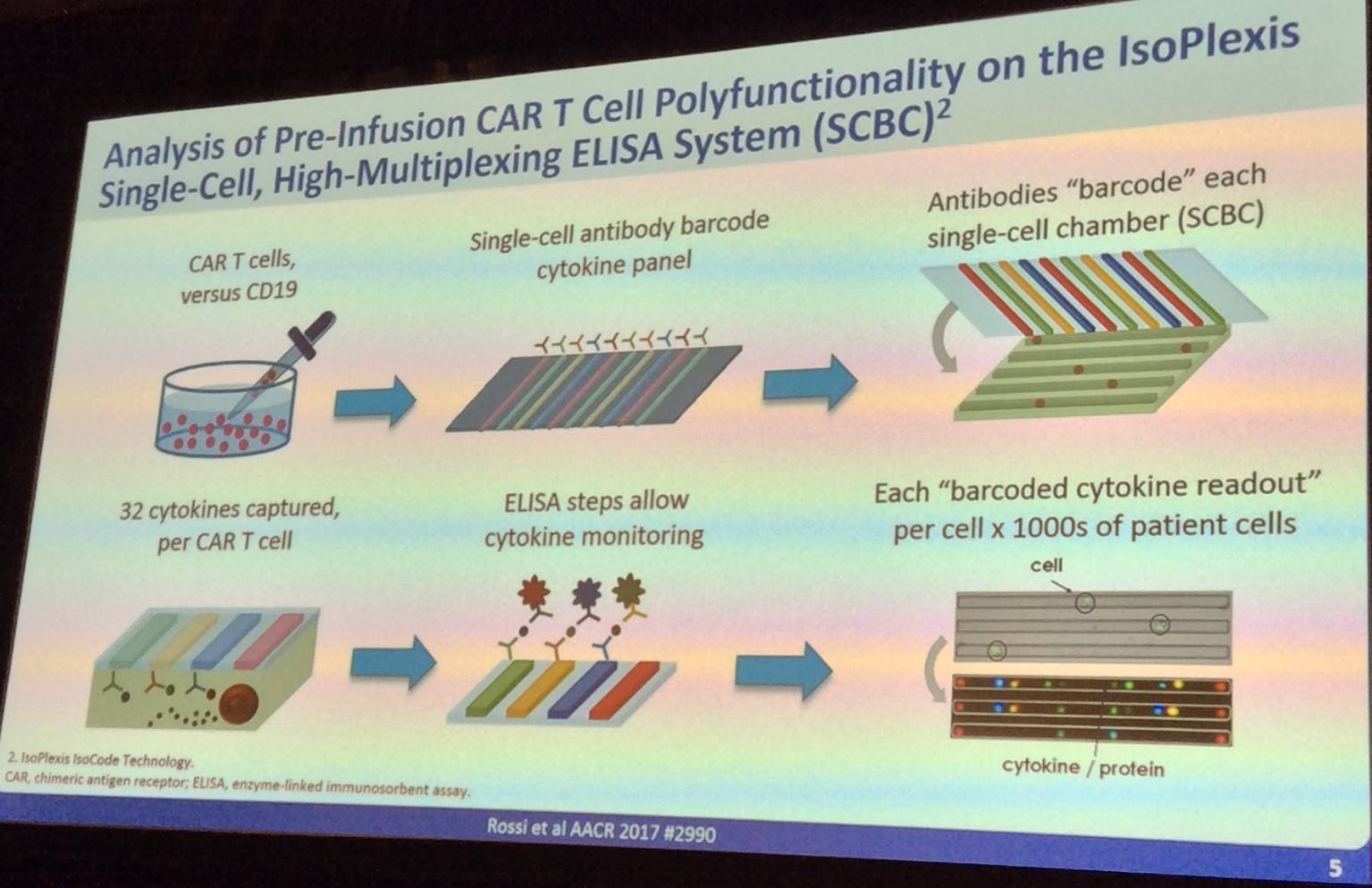
Rossi then went on to outline the recent results published by Kochenderfer et al. in the Journal of Clinical Oncology (NCT00924326), which found that anti-CD19 CAR T-cell therapy results in durable tumor regression in relapsed NHL patients:
- Total number of patients = 22 (n = 19 DLBCL; n = 2 FL; n = 1 MCL)
- Low dose conditioning therapy; 1–2x106 CAR+ T-cells/kg
- Clinical response:
- ORR = 73%; CR = 55%; PR = 18%
- CRs ongoing = 11/12 (7–24 months+)
- Toxicities
- ≥Grade 3 neurologic events = 55%
- ≥Grade 3 hypotension by CTCAE3 = 18%
- All toxicities completely resolved, most within 2 weeks
Following this, Rossi presented data indicating that pre-infusion single-cell CAR PSI and CAR PEAK levels in vivo and polyfunctional strength are significantly associated with objective response. However, pre-infusion product PSA is not significantly associated with expansion of CAR T-cells.
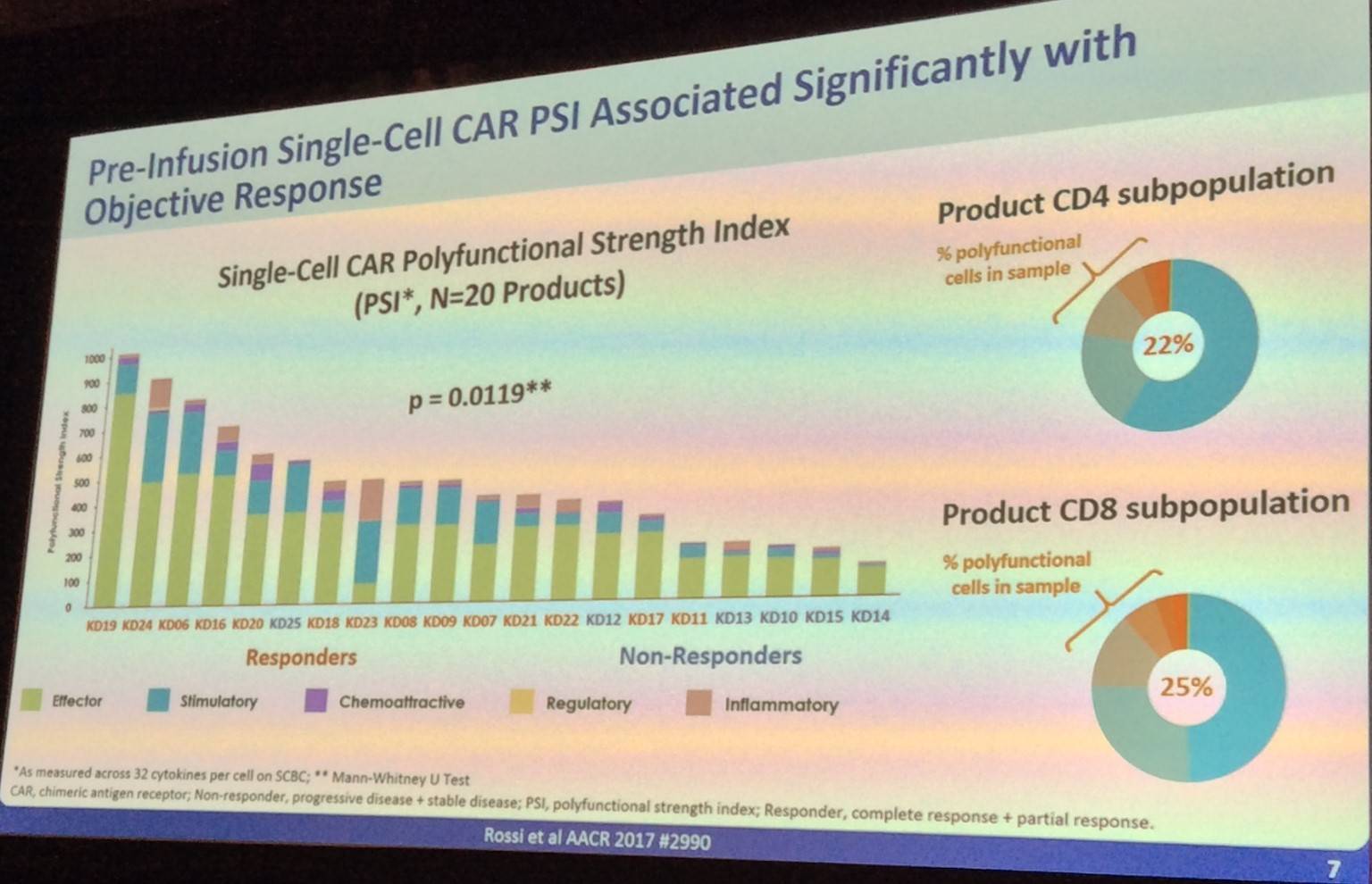
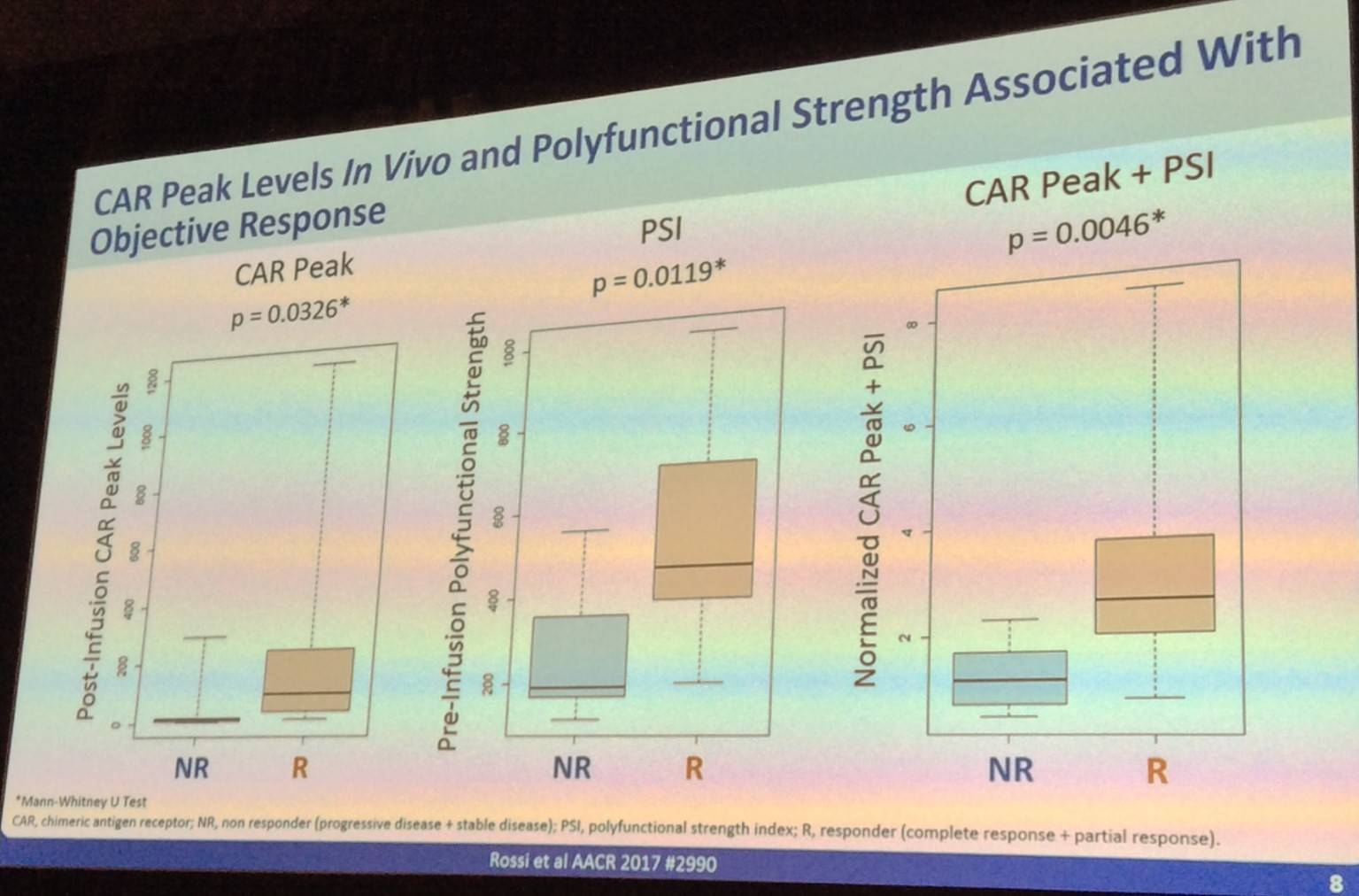
Before concluding the talk, John Rossi also presented data which indicated that IL-17a, IFN-γ, IL-8, IL-5, and MIP-1α drive CD4 polyfunctionality in responders.

- Rossi J. Polyfunctional anti-CD19 CAR T cells determined by single-cell multiplex proteomics associated with clinical activity in patients with advanced non-Hodgkin’s lymphoma [Abstract]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; 2017. Abstract nr [2990].
More about...

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








