All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
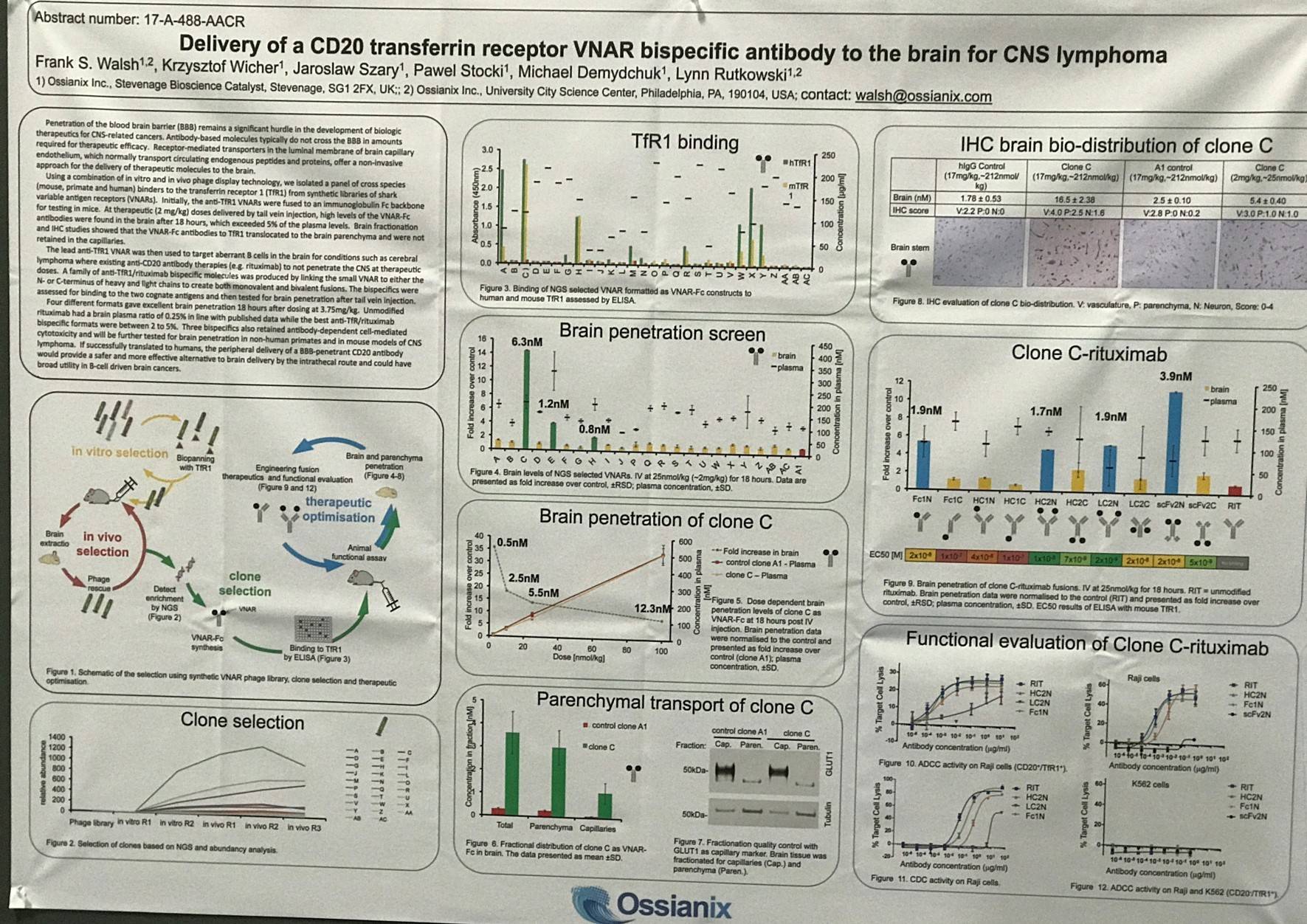
AACR 2017 | Poster 3631/4 – Delivery of a CD20 transferrin receptor VNAR bispecific antibody to the brain for Central Nervous System Lymphoma
Bookmark this article
At the American Association for Cancer Research (AACR) annual meeting in Washington, DC, USA, on Tuesday 4th April, a poster session titled “BITES Bispecifics and Checkpoints” took place.
One of the posters on display (3631 / 4) was titled “Delivery of a CD20 transferrin receptor VNAR bispecific antibody to the brain for CNS lymphoma” by Frank S. Walsh from Ossianix, Philadelphia, PA, and colleagues.
Currently, the challenge facing the development of biologic therapies for CNS-related cancers is getting the agent to cross the blood brain barrier.
This group used in vitro and in vivo phage display technology; they identified a panel of cross species (rodent, primate, and human) binders to the Transferrin Receptor 1 (TfR1) from synthetic libraries of shark Variable Antigen Receptors (VNARs).
Key Highlights:
- To begin with, the anti-TfR1 VNARs were attached to an Ig Fc backbone for testing in mice
- When therapeutic doses (2mg/kg) were administered via tail vein injection, high levels of VNAR-Fc antibodies were reported in the brain after 18 hours, >5% of the plasma levels
- Brain fractionation studies indicated that VNAR-Fc antibodies to TfR1 translocated to the brain parenchyma and were not retained in capillaries
- The lead anti-TfR1 VNAR was used to target aberrant B-cells in the brain for diseases like cerebral Lymphoma
- A family of anti-CD20/TfR1 bispecific molecules were produced; small VNAR were linked to either the N- and C- terminus of heavy and light chains to generate monovalent and bivalent fusions
- The anti-CD20 agent used was rituximab
- 18 hours after dosing at 3.75mg/kg, ≥4 different formats gave outstanding brain penetration
- Unmodified rituximab brain plasma ratio = 0.25; the best anti-TfR1-rituximab bispecific formats = between 2 to 5%
- The bispecific formats maintain Antibody-Dependent Cell Cytotoxicity (ADCC)
The poster concluded by stating that these bispecific formats will be investigated further for brain penetration in non-human primates and in CNS Lymphoma rodent models.
If these results are successfully replicated in larger animals, the delivery of the bispecific CD20/TfR1 antibody would present as a safer and more effective substitute to intrathecal delivery of CD20 antibody and could have wide range of uses to treat and manage B-cell driven brain cancers.
- Walsh F.S. et al. Delivery of a CD20 transferrin receptor VNAR bispecific antibody to the brain for CNS lymphoma [Poster]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; 2017. Poster nr [3631 / 4].
More about...

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








