All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
An expert panel hosted by

Sequencing immune-based therapies in B-cell malignancies
with Ulric Jäger, Sagar Lonial, and Krina Patel

Saturday, June 15 | 18:00-19:30 CEST
Register nowThis independent education activity is sponsored by Bristol Myers Squibb. All content is developed independently by the faculty. Funders are allowed no direct influence on the content of this activity.
The Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
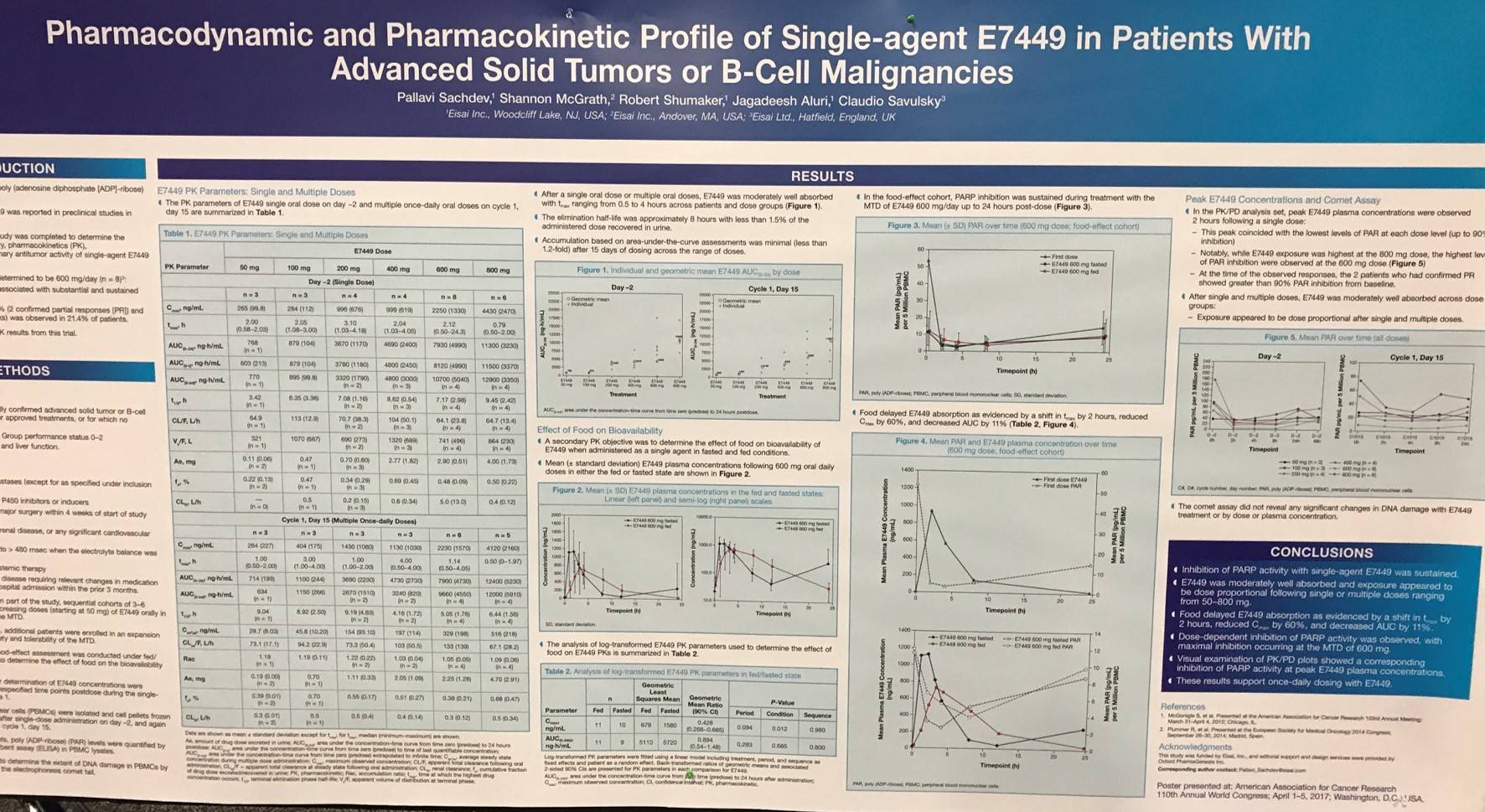
AACR 2017 | poster 5050/25 – pharmacodynamic and pharmacokinetic relationship of single agent E7449 in patients with relapsed/refractory Non-Hodgkin Lymphoma
Bookmark this article
At the American Association for Cancer Research (AACR) annual meeting in Washington, DC, USA, on Wednesday 5th April, a poster session titled “Anticancer Precision Clinical Pharmacology” took place.
One of the poters on display (5050 / 25) was titled “Pharmacodynamic and pharmacokinetic relationship of single agent E7449 in patients with advanced solid tumors or B-cell malignancies” by Pallavi Sachdev from Eisai, Inc., Woodcliff Lake, NJ, and colleagues.
E7449 is a small-molecule inhibitor of Poly (ADP-Ribose) Polymerase (PARP). A multicenter, open-label, phase I study was conducted aiming to identify the Maximum Tolerated Dose (MTD), safety, Pharmacokinetics (PK), Pharmacodynamics (PD), and preliminary anti-tumor activity of single-agent E7449 (NCT01618136). In the poster, the group presented additional PD and PK results from the phase I trial.
Patients (n = 28) were 18 years or older and had measurable, confirmed, advanced solid tumors or B-Cell Lymphoma that had progressed after approved treatment. Those with Lymphoma must have R/R disease that progressed following three previous systemic treatment regimens. Eligible subtypes were MCL, MZL, FL, DLBCL, and CLL. Patients received E7449 at 50, 100, 200, 600, or 800mg/day. An expansion cohort (n = 13) was used to study food. PD assessments included measurement of PARP activity and comet assay to determine the extent of DNA damage.
Key Highlights:
- MTD = 600mg/day (n = 8)
- At this dose level, treatment-emergent AEs resulted in withdrawal of E7449 in 1 pt and dose interruption in 2 pts
- Treatment at the MTD was associated with substantial and sustained dose-dependent PARP inhibition
- ORR = 7.1% (PR = 2); durable SD (≥23 weeks) reported in 21.4% of pts
- Food-effect cohort: PARP inhibition (≤90%) was sustained during treatment at the MTD up to 24 hours post-dose
- Peak E7449 plasma concentrations delayed by 2 hours in fed versus fasted pts
- PK/PD analysis set: peak E7449 plasma concentrations observed at 2 hours after single dose and corresponded with the lowest levels of Poly (ADP-Ribose) (PAR; ≤90% inhibition)
- E7449 exposure was highest at 800mg dose, however the lowest PAR levels occurred at 600mg dose
- At the time of observed responses, the 2 pts with confirmed PR demonstrated >90% PAR inhibition from baseline
- E7449 did not affect the level of DNA damage detected by the comet assay; DNA damage levels remained similar to those in healthy donors
The poster concluded that “these results support E7449 dosing at 600mg/day.”
- Sachdev P. et al. Pharmacodynamic and pharmacokinetic relationship of single agent E7449 in patients with advanced solid tumors or B-cell malignancies [Poster]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; 2017. Poster nr [5050 / 25].

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








