All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
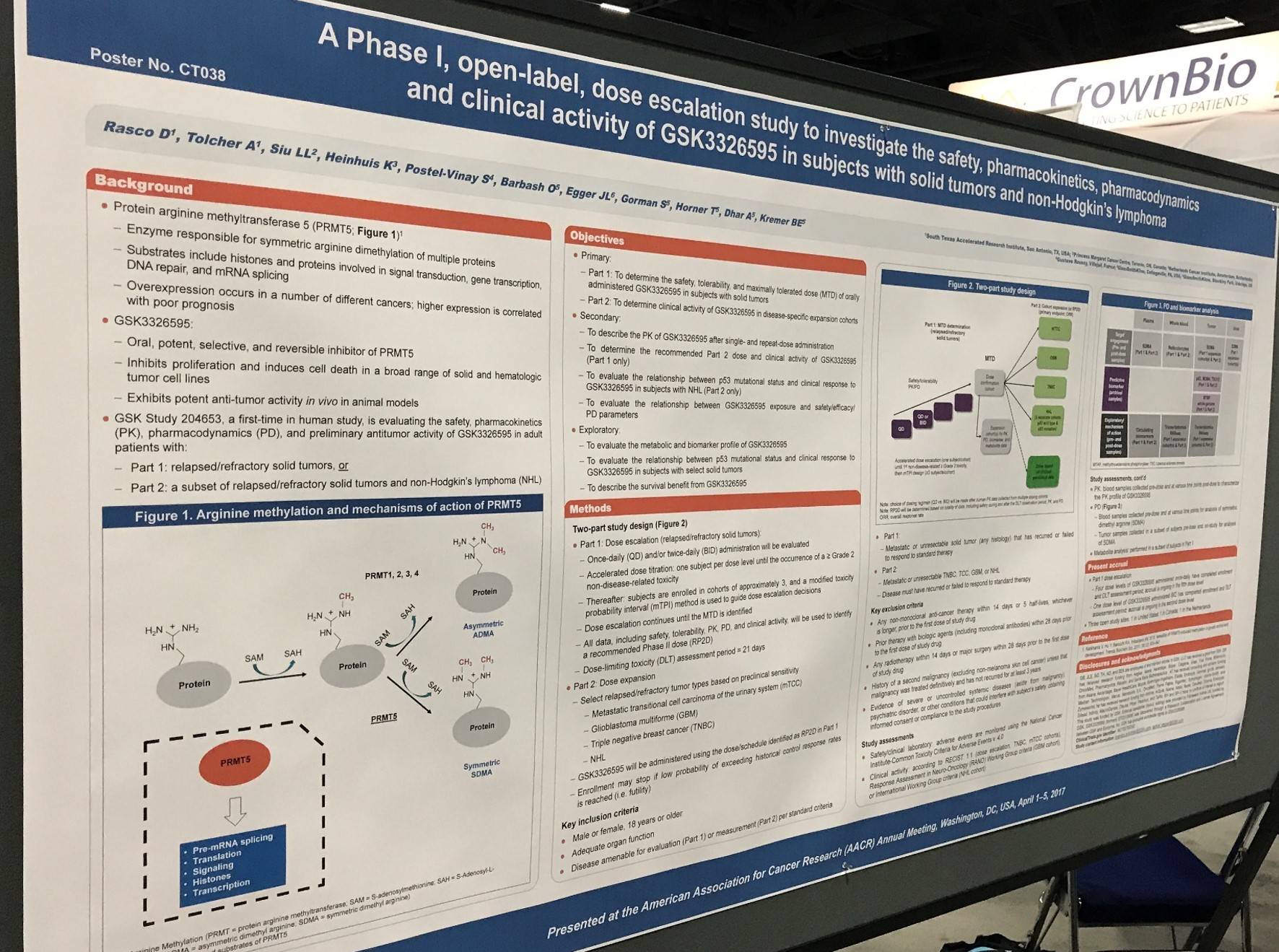
AACR 2017 | Poster CT038/14 – 204653 study design: a phase I, open-label, dose-escalation study of GSK3326595 in patients with Non-Hodgkin Lymphoma
Bookmark this article
This year’s American Association for Cancer Research (AACR) annual meeting took place on 1–5 April in Washington, DC, USA. The program committee Chair was Kornelia Polyak, MD, PhD, from the Dana-Farber Cancer Institute, Boston, Massachusetts.
On Monday 3rd April, a poster (CT038 / 14) by Drew Rasco, from South Texas Accelerated Research Therapeutics, San Antonio, TX, et al. titled “A phase I, open-label, dose-escalation study to investigate the safety, pharmacokinetics, pharmacodynamics, and clinical activity of GSK3326595 in subjects with solid tumors and non-Hodgkin's lymphoma” was presented.
GSK3326595 is an orally administered, selective, and reversible Protein Arginine Methyltransferase 5 (PRMT5) inhibitor, which halts proliferation and induces apoptosis in numerous solid and hematologic tumor cell lines. It has been shown to have potent anti-tumor activity in vivo in animal models.
Key Highlights:
Design
- Two-part study design; Part 1 is a dose escalation in R/R solid tumors and Part 2 is a dose expansion in R/R solid tumors and NHL
- In Part 2, GSK3326595 will be given according to the Recommended Phase II Dose (RP2D) defined in Part 1
- Enrollment to Part 2 may potentially be halted if “low probability of exceeding historical control response rates is reached (i.e. futility)”
Enrollment criteria
- Gender: male or female
- Age: ≥18 years
- Disease measured per standard criteria (for Part 2)
- Part 2: metastatic or unresectable NHL; disease must have recurred or failed to respond to standard therapy
Present accrual
- Part 1
- As of March 24 2017, no DLTs reported in first 4 dose levels of monotherapy
- Enrollment for dose level 5 cohort is ongoing
- Pk/PD cohort has been initiated
- Open study sites (n = 10) in the US, Spain, Italy, France, The Netherlands, Australia, and Canada
- Rasco D. et al. A phase I, open-label, dose-escalation study to investigate the safety, pharmacokinetics, pharmacodynamics, and clinical activity of GSK3326595 in subjects with solid tumors and non-Hodgkin's lymphoma [Poster]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; 2017. Poster nr [CT038 / 14].
More about...

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








