All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
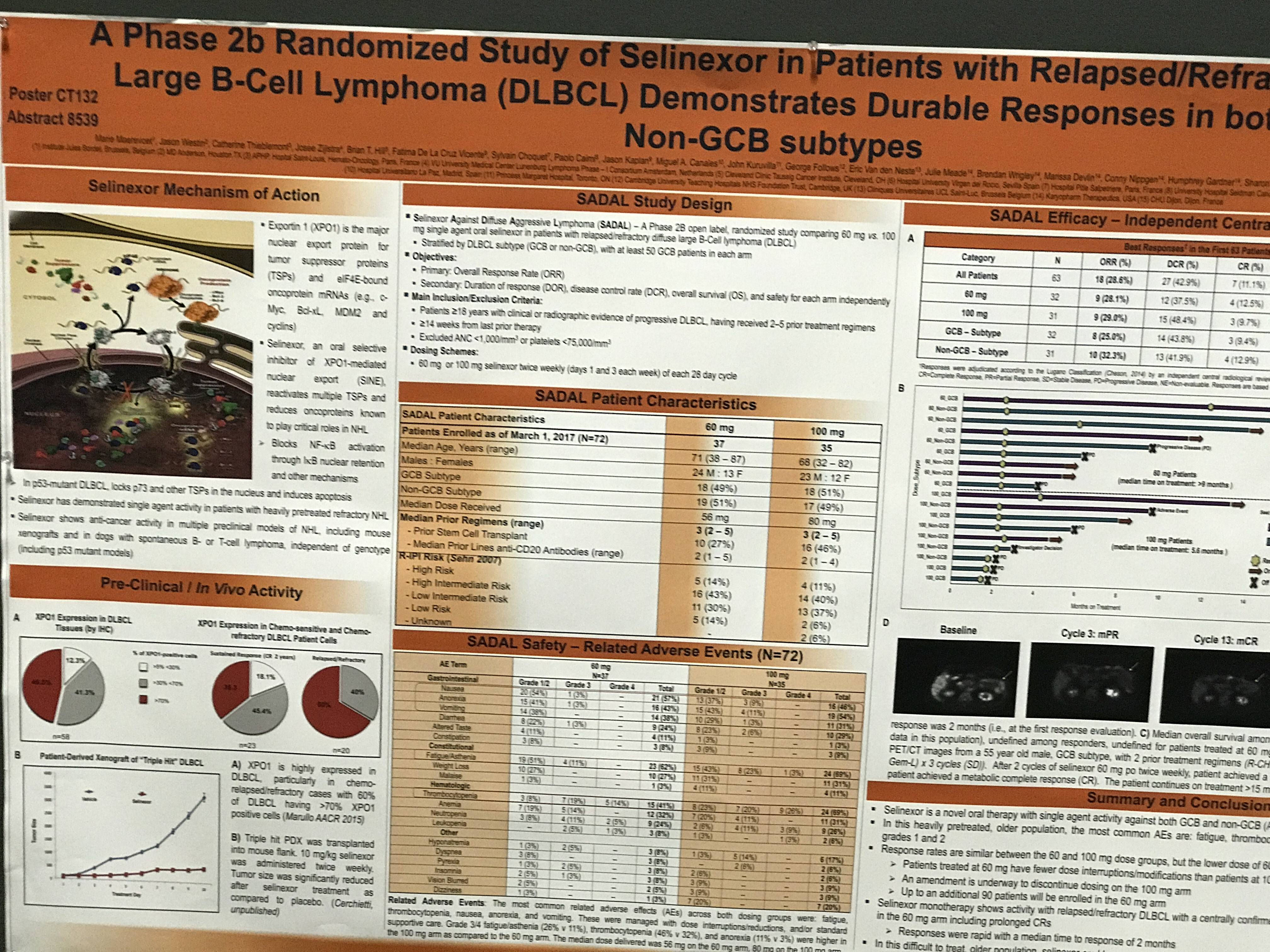
AACR 2017 | Poster CT132/13 – A phase IIb randomized study (SADAL) of selinexor achieves durable responses in GCB & Non-GCB subtypes of relapsed/refractory Diffuse Large B-Cell Lymphoma
Bookmark this article
At the American Association for Cancer Research (AACR) annual meeting in Washington, DC, USA, on Tuesday 4th April, a poster session titled “Phase I-III Clinical Trials and Pediatric Clinical Trials” took place.
One of the posters on display (CT132 / 13) was titled “A Phase 2b randomized study of selinexor in patients with relapsed/refractory Diffuse Large B-Cell Lymphoma (DLBCL) demonstrates durable responses in both GCB & Non-GCB subtypes” by Marie Maerevoet from Institut Jules Bordet, Brussels, Belgium, and colleagues.
In this phase IIb trial (SADAL), R/R DLBCL patients were randomized to receive 60mg or 100mg of selinexor twice weekly (8 doses) per 28-day cycle. Additionally, patients were grouped by the subtype of their DLBCL (GCB or non-GCB). The primary objectives are to determine the ORR, and safety of 60mg compared to 100mg doses.
Key Highlights:
- In total, 67 pts enrolled (n = 33 at 60mg; n = 34 at 100mg) enrolled
- The most common related AEs across both dosing groups (grade 1/2) = nausea (46%), anorexia (43%), vomiting (36%), and fatigue (34%)
- Frequent grade 3/4 AEs = thrombocytopenia (39%), fatigue (18%), neutropenia (16%), and anemia (10%)
- These were managed with dose interruption/reduction, platelet stimulators, and/or standard supportive care
- Grade 3/4 fatigue was higher in 100mg arm (13%) vs the 60mg arm (4%)
- Grade 3/4 thrombocytopenia was higher in 100mg arm (22%) vs the 60mg arm (16%)
- Among 65 evaluable pts, ORR = 21.5%
- Responders had a median of 3 prior treatment regimens
- CR = 12.3% (n = 8); PR = 9.2% (n = 6)
- Remain on treatment = 9 responders, including 7 CRs
- Median time on study for CR is 8.8+ months
- ORR by subtype: GCB = 23.5%; non-GCB = 19.3%
- ORR was higher in 60mg arm (26.4%) vs 100 mg arm (16.1%), potentially because of better tolerability and less time without drug exposure
In conclusion, monotherapy with selinexor demonstrates anti-cancer activity in R/R DLBCL patients including those with GCB subtype. Dosing at 60mg twice weekly was tolerated better than 100mg twice weekly, with less interruptions to dosing due to toxicity and a trend towards higher response rates. Moreover, durable objective responses were achieved with selinexor, which the authors hypothesize may be “associated with clinical benefit.”
- Maerevoet M. et al. A Phase 2b randomized study of selinexor in patients with relapsed/refractory Diffuse Large B-Cell Lymphoma (DLBCL) demonstrates durable responses in both GCB & Non-GCB subtypes [Poster]. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; 2017. Poster nr [CT132 / 13].

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








