All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Brentuximab vedotin plus RCHP appears an effective and well tolerated frontline therapy in high-intermediate/high-risk DLBLC expressing CD30
Bookmark this article
On 3–6 December 2016 in San Diego, CA, the 58th Annual Meeting & Exposition of the American Society of Hematology (ASH) took place.
On Saturday 3rd December, an oral abstract session was held between 9:30am and 11:00am in the “Aggressive Lymphoma (DLBCL and Other Aggressive B-Cell NHLs) – Results from Retrospective/Observational Studies Program” category. This session was moderated by Oreofe Odejide, MD, MPH, of the Dana-Farber Cancer Institute, and Carla Casulo, MD, of the University of Rochester Medical Center.
Abstract #104 was presented during this session, titled “Results of an Ongoing Phase 2 Study of Brentuximab Vedotin with Rchp As Frontline Therapy in Patients with High-Intermediate/High-Risk Diffuse Large B Cell Lymphoma (DLBCL)” by Lihua E. Budde, MD, PhD, of the Hematology & Hematopoietic Cell Transplantation department, City of Hope National Medical Center, Duarte, CA, and colleagues.
This ongoing study is split into parts. Part 1 included patients with high-intermediate or high-risk DLBCL who were administered 1.2 or 1.8mg/kg brentuximab vedotin plus RCHOP. However, 3 of the initial 10 patients treated at the 1.8mg/kg dose level developed grade 3 peripheral neuropathy and so all further patients enrolled received treatment at the lower dose of 1.2mg/kg brentuximab vedotin plus RCHOP. After enrollment was completed for Part 1, a protocol amendment took place for a non-randomized portion of the study (Part 2; NCT01925612) aimed at determining the efficacy and safety of 6 cycles 1.8mg/kg brentuximab vedotin plus RCHP. Part 3 is an open-labelled, randomized study comparing brentuximab vedotin + RCHP to RCHOP. This presentation included initial results from Part 2 and updated results from Part 1.
Part 2
- At time of analysis, 11 patients were treated (7 males, 4 female, 22–78 years of age)
- ORR = 91% (9 CRs, 1 PR); progressive disease occurred in one patient after Cycle 4
- The most common (>20%) treatment-emergent AEs were alopecia (73%), nausea (73%), fatigue (64%), constipation (55%), peripheral sensory neuropathy (55%), neutropenia (36%), throat irritation (36%), chills (27%), diarrhea (27%), headache (27%), and stomatitis (27%)
- Serious AEs occurred in 5 patients and included febrile neutropenia, bacteremia, nausea, pneumocystis jiroveci pneumonia, pulmonary embolism, and vomiting
- Peripheral sensory neuropathy occurred in 6 patients; all grade 1 or 2
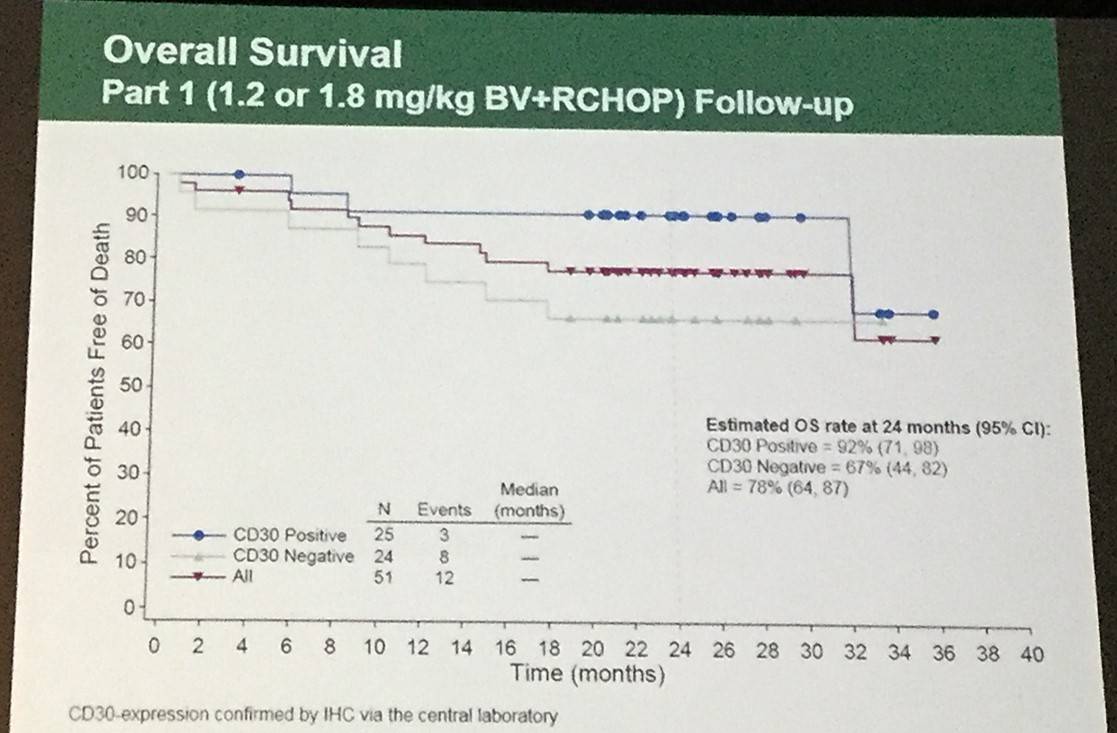 Part 1
Part 1
- 18-month PFS in patients with CD30 expression (n=25) or without detectable CD30 expression (n=24) by IHC was 79% (95% CI: 57%, 91%) versus 58% (95% CI: 36%, 75%), respectively
- OS in CD30 detectable versus CD30 undetectable was 92% (95% CI: 71%, 98%) versus 67% (95% CI: 44%, 82%), respectively
- Treatment-emergent peripheral neuropathy was reported in 38/51 patients (75%) who were administered brentuximab vedotin plus RCHOP
- Resolution of all or some of the peripheral neuropathy occurred in 21/38 patients (55%)
 It was concluded that 1.8mg/kg brentuximab vedotin plus RCHP is an effective frontline therapy in high-intermediate/high-risk DLBCL expressing CD30 and appears well tolerated.
It was concluded that 1.8mg/kg brentuximab vedotin plus RCHP is an effective frontline therapy in high-intermediate/high-risk DLBCL expressing CD30 and appears well tolerated.
Abstract
Introduction: DLBCL is the most common lymphoid neoplasm in adults (Swerdlow 2016). While durable CRs are achieved in approximately 70% of patients (pts) with frontline RCHOP therapy (Pfreundschuh 2008), pts with high-risk features often experience disease resistance or relapse. In Part 1 of an ongoing study, pts with high-intermediate or high risk DLBCL by international prognostic index (IPI) scores, regardless of CD30 expression by IHC, were treated with 1.2 or 1.8 mg/kg brentuximab vedotin (BV) combined with RCHOP. After 3 of the first 10 pts treated at 1.8 mg/kg BV+RCHOP developed Grade 3 peripheral neuropathy (per Standardized MedDRA Query [SMQ]), all pts enrolled subsequently received treatment with 1.2 mg/kg BV+RCHOP. Following completion of enrollment in Part 1, the protocol was amended to enroll a non-randomized portion of the study (Part 2) evaluating the safety and efficacy of 1.8 mg/kg BV+RCHP (Yasenchak 2015), followed by an open-label, randomized portion comparing BV+RCHP to RCHOP (Part 3). Initial results from Part 2 and updated results from Part 1 are reported here.
Methods: For Part 2 of the study, pts with CD30-expressing high-intermediate and high-risk DLBCL were treated with up to 6 cycles of 1.8 mg/kg BV+RCHP (NCT01925612). Key inclusion criteria were CD30 expression by IHC performed by a local pathology lab and standard IPI scores of 3–5 or age-adjusted IPI (aaIPI) scores of 2–3 (high-intermediate/high risk). CD30 expression was confirmed by a central pathology lab, although CD30 expression by local pathology lab was required for eligibility. Disease response was evaluated with PET/CT per Cheson 2007.
Results: At the time of analysis for this ongoing study, 11 pts in Part 2 were treated with BV+RCHP (7 male, 4 female; 22‑78 yrs). Of these pts, 9 had high-intermediate risk (IPI 3, aaIPI 2) and 2 had high risk disease (IPI 4-5, aaIPI 3), 6 had Stage IV disease, and 6 had an ECOG score of 2. At the end of treatment, the overall response rate was 91% (9 CR, 1 PR); 1 pt had PD after Cycle 4. The most frequent (>20%) treatment-emergent adverse events (AEs) were alopecia and nausea (73% each); fatigue (64%); constipation and peripheral sensory neuropathy (55% each); neutropenia and throat irritation (36% each); and chills, diarrhea, headache, and stomatitis (27% each). Grade 3 or 4 AEs occurred in 8 pts and 5 pts had serious AEs, which included febrile neutropenia, bacteremia, nausea, pneumocystis jiroveci pneumonia, pulmonary embolism, and vomiting. Peripheral sensory neuropathy occurred in 6 pts and all were Grade 1 or 2 events; no peripheral motor neuropathy AEs were reported. No AEs were fatal or led to discontinuation. One pt discontinued treatment after Cycle 4 due to disease progression.
For the first 51 pts in Part 1, the progression-free survival (PFS) at 18 months for pts with CD30 expression (25 pts) or without detectable CD30 expression (24 pts) by IHC was 79% (95% CI: 57%, 91%) versus 58% (95% CI: 36%, 75%), respectively. Overall survival for pts was 92% (95% CI: 71%, 98%) versus 71% (95% CI: 48%, 85%), respectively. Ten pts had pre-existing peripheral neuropathy (per SMQ) at study entry. Treatment-emergent peripheral neuropathy (per SMQ) was observed in 75% of pts (38/51) who received BV+RCHOP; 55% of these pts (21/38) had resolution of all or some peripheral neuropathy events.
Conclusions: 1.8 mg/kg BV+RCHP is active as frontline treatment in CD30-expressing, high-intermediate/high risk DLBCL. When combined with RCHP, 1.8 mg/kg BV appears to be well-tolerated. The PFS and OS for pts with CD30-expression who received BV+RCHOP appear promising. The study is currently ongoing in pts with CD30-expressing high-intermediate/high risk DLBCL to assess the safety and activity of 1.8 mg/kg BV+RCHP versus standard RCHOP.
- Budde E.L. et al. Results of an Ongoing Phase 2 Study of Brentuximab Vedotin with Rchp As Frontline Therapy in Patients with High-Intermediate/High-Risk Diffuse Large B Cell Lymphoma (DLBCL). Oral Abstract #104: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








