All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Intensive chemotherapy with DA-EPOCH-R presents new frontline treatment option in patients with high-risk DLBCL
Bookmark this article
On 3–6 December 2016 in San Diego, CA, the 58th Annual Meeting & Exposition of the American Society of Hematology (ASH) took place.
On Saturday 3rd December, an oral abstract session was held between 9:30am and 11:00am in the “Aggressive Lymphoma (DLBCL and Other Aggressive B-Cell NHLs) – Results from Retrospective/Observational Studies Program” category. This session was moderated by Oreofe Odejide, MD, MPH, of the Dana-Farber Cancer Institute, and Carla Casulo, MD, of the University of Rochester Medical Center.
Abstract #106 was presented during this session, titled “High Risk Diffuse Large B Cell Lymphoma: A Comparison of Aggressive Subtypes Treated with Dose Adjusted Chemotherapy – the University of Texas MD Anderson Experience” by Vishwanath Sathyanarayanan, MD, of the Department of Lymphoma/Myeloma at the MD Anderson Cancer Center, Houston, TX, and colleagues.
This abstract presents results of a retrospective review of all consecutive, treatment naïve patients with DLBCL treated with (DA) EPOCH-R at the MD Anderson Cancer Center between 2010 and 2014. The aim was to evaluate the demographic, prognostic and treatment variables compared to clinical response and survival outcomes in three subgroups:
- DHL (GCB; n=22)
- DLBCL without MYC and BCL2 expression (GCB; n=46)
- DEL (GCB and non-GCB; n=16)
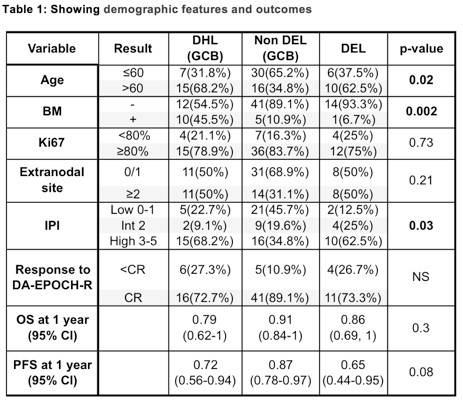
Two-hundred and thirty-three high-risk patients with DLBCL, treated with (DA) EPOCH-R, were identified. Patients in the DHL were more likely to have bone marrow involvement, and the DEL and DHL groups were more commonly aged over 60 years and had higher IPI scored compared to the non DEL GCB group. 1-year OS, PFS and CR rate were not significantly different between the three subgroups (figure 1).
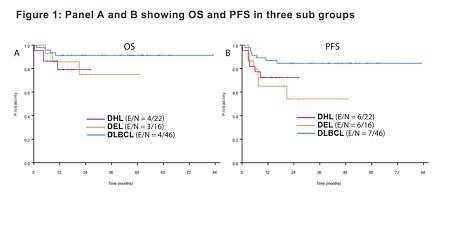
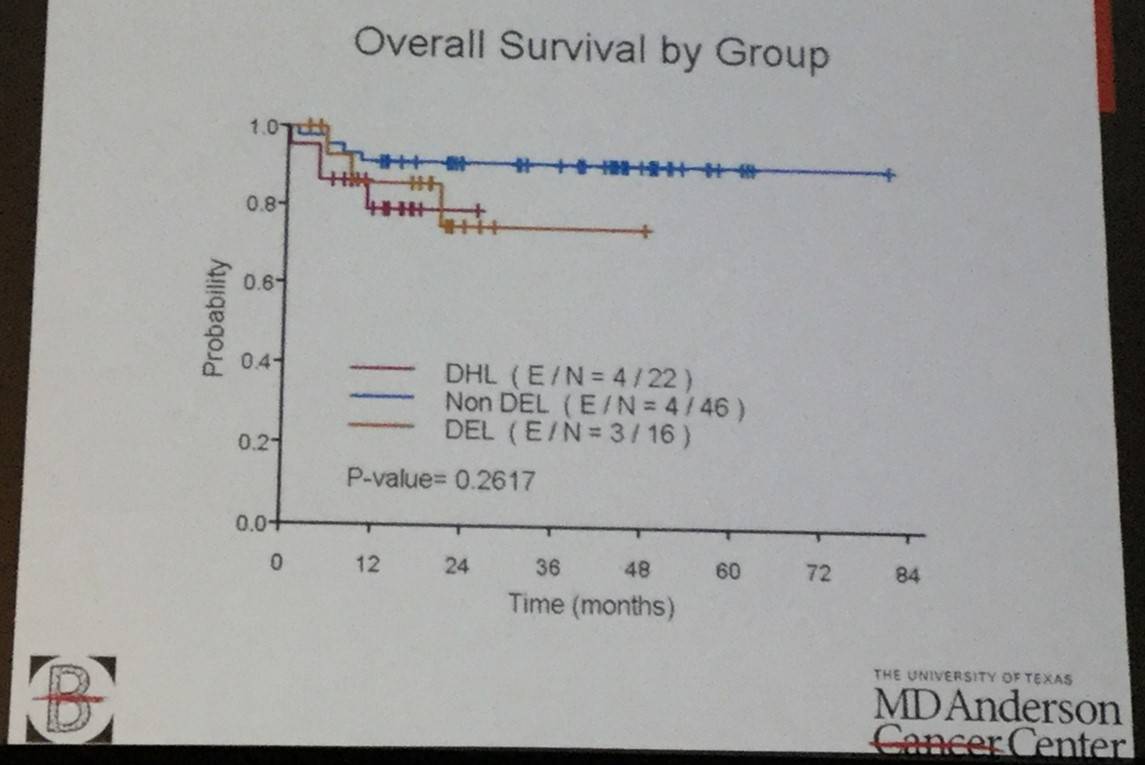
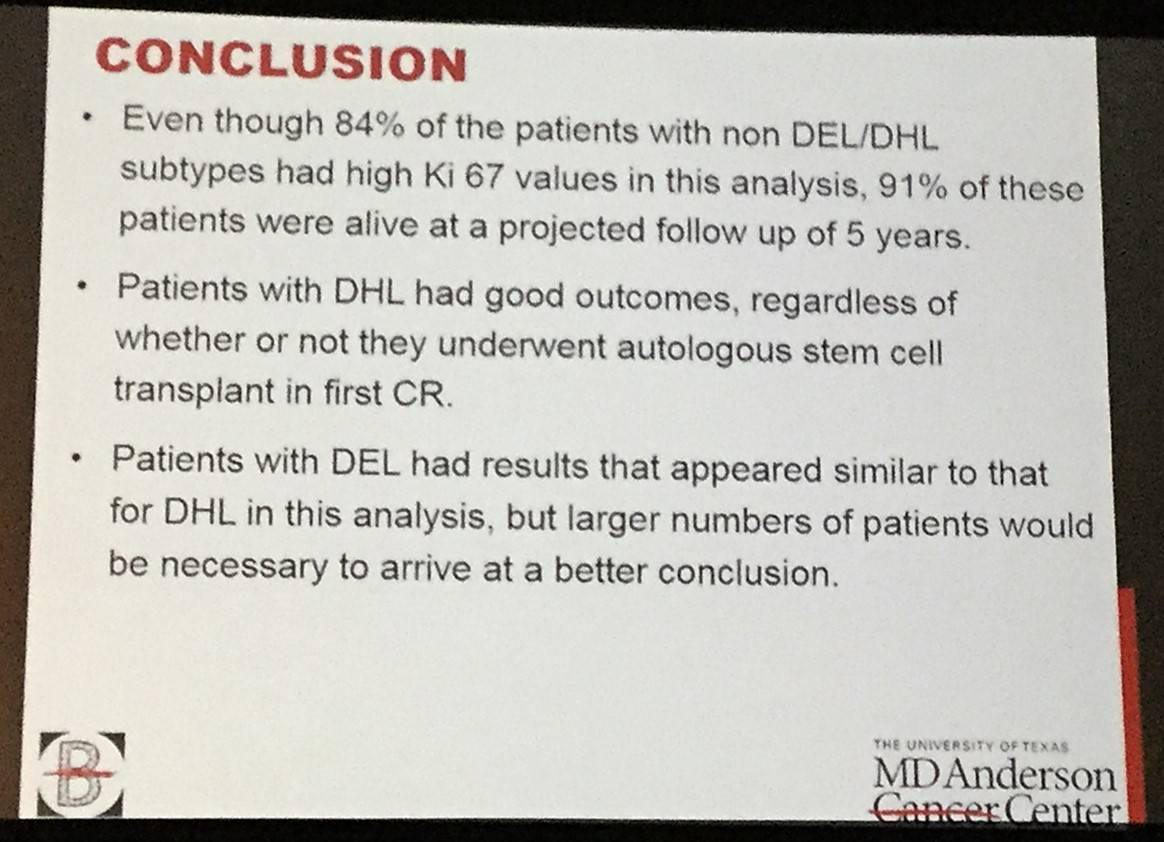
This talk concluded by stating that the results of this retrospective study indicated that OS in DHL, DEL and non DEL DLBCL are not statistically different and so intensive chemotherapy could be considered as an alternative frontline treatment option for patients with high-risk DLBCL. However, future randomized trials need to be conduction to confirm this.
Abstract
Background: Diffuse large B cell lymphoma (DLBCL) is the most common type of non Hodgkin lymphoma (NHL). Nearly 50% of high-risk DLBCL patients are not cured with standard rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (RCHOP). High risk DLBCL may be defined as double hit lymphoma (DHL, translocation of MYC and BCL2 or BCL6), double expressor lymphoma (DEL, over expression of MYC and BCL2), high risk international prognostic index (IPI) of 3-5, high Ki-67, and non-germinal center subtype (non-GCB). The majority of DHL cases occur in the GCB subtype, as opposed to the majority of DEL cases which occur in non-GCB. Hence we sought to compare different high risk subsets treated with dose-adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone and rituximab (DA) EPOCH-R. In single arm phase II clinical trials, dose adjusted (DA) EPOCH-R has shown promising results, with potential greater efficacy in the GCB subtype in subset analyses (Wilson et al, Hematologica 2012). A randomized phase III study comparing RCHOP with (DA) EPOCH-R in newly diagnosed DLBCL has completed accrual, with highly anticipated results due in late 2016.
Methods: We conducted a retrospective review of all consecutive, newly diagnosed DLBCL patients treated with (DA) EPOCH-R at MD Anderson Cancer Center from 2010 to 2014. Eligible patients were 18 years or greater, had high-risk DLBCL as determined by the treating physician, and had available data of treatment and response. The cell of origin subtype was determined by immunohistochemistry using Hans algorithm, and MYC and BCL2 positivity were defined as BCL2 positive in at least 70% and MYC positive in at least 40% of cells. DHL was defined as rearrangement of MYC and BCL2 or BCL6 by fluorescent in situ hybridization. The objectives were to analyze demographic, prognostic, and treatment variables in comparison with clinical response and survival outcomes in three sub groups which included 1. DHL (GCB) 2. DLBCL without MYC and BCL2 expression (GCB), and 3. DEL (GCB and non GCB). Complete response (CR), overall survival (OS) and progression free survival (PFS) were calculated using standard methods. Statistical analysis was done using Fishers exact test or Chi-square test / Kruskal-Wallis test. Kaplan-Meier method was used for time-to-event analysis including overall survival and progression free survival. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups.
Results: We identified 233 high risk DLBCL patients treated with (DA) EPOCH-R. After filtering the data to identify patients which were included in our three groups, we identified 22 patients with DHL (GCB), 46 patients with non DEL (GCB), and 16 with DEL. The demographic features and outcomes are mentioned in the table 1 below. The DHL group had more frequent bone marrow (BM) involvement, and the DHL and DEL groups were more frequently age >60 years and high IPI in comparison to the non DEL GCB group. The CR rate, OS and PFS at 1 year were not significantly different between these three groups. Figure 1 highlights the OS (A) and PFS (B) results of each group.
Conclusions: (DA) EPOCH-R is highly effective in patients with subsets of patients with high-risk DLBCL and may be able to overcome prognostic factors which have been shown to be adverse with RCHOP therapy. The results of this retrospective study suggest that OS in DHL, DEL and non DEL (GCB) are not statistically different. Hence, intensive chemotherapy with (DA) EPOCH-R could be considered as a frontline treatment option for patients with high risk DLBCL, pending further confirmation in randomized trials.

- Sathyanarayanan V. et al. High Risk Diffuse Large B Cell Lymphoma: A Comparison of Aggressive Subtypes Treated with Dose Adjusted Chemotherapy – the University of Texas MD Anderson Experience. Oral Abstract #106: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Interpretation of R2-CHOP in large cell lymphoma
Interpretation of R2-CHOP in large cell lymphoma
Jun 24, 2019
Interpretation of R2-CHOP in large cell lymphoma
Interpretation of R2-CHOP in large cell lymphoma
Jun 24, 2019
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








