All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Brentuximab vedotin and ESHAP is a highly effective combination for remission induction in pre-APBSCT patients with R/R HL
Bookmark this article
The Annual Meeting & Exposition of the American Society of Hematology (ASH) took place in San Diego, CA, on 3–6 December 2016.
On Monday 5th December, an oral abstract session was held between 4:30pm and 6:00pm in the “Hodgkin Lymphoma and T/NK Cell Lymphoma – Clinical Studies Program: Oral and Poster Abstracts” category. This session was moderated by Stephen Ansell, MD, PhD, of the Mayo Clinic, and Anas Younes, MD, of the University of Texas M.D. Anderson Cancer Center.
Abstract #1109 was presented during this session, titled “Brentuximab vedotin plus ESHAP (BRESHAP) is a Highly Effective Combination for Inducing Remission in Refractory and Relapsed Hodgkin Lymphoma Patients Prior to Autologous Stem Cell Transplant: A Trial of the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO)” by Ramon Garcia-Sanz, MD, PhD, of the Hospital Universitario de Salamanca, Spain, and colleagues.
This abstract presented data from a phase II trial (NCT02243436) aiming to assess the response rate with brentuximab vedotin and ESHAP chemotherapy as second-line treatment for Relapsed/Refractory (R/R) Hodgkin Lymphoma (HL) prior to Autologous Peripheral Blood Stem Cell Transplantation (APBSCT). The primary endpoint of this trial was the proportion of Complete Responses (CRs) pre-APBSCT. The number of patients enrolled was 66; 35 females and 31 males, with a median age of 36 (18–66 years).
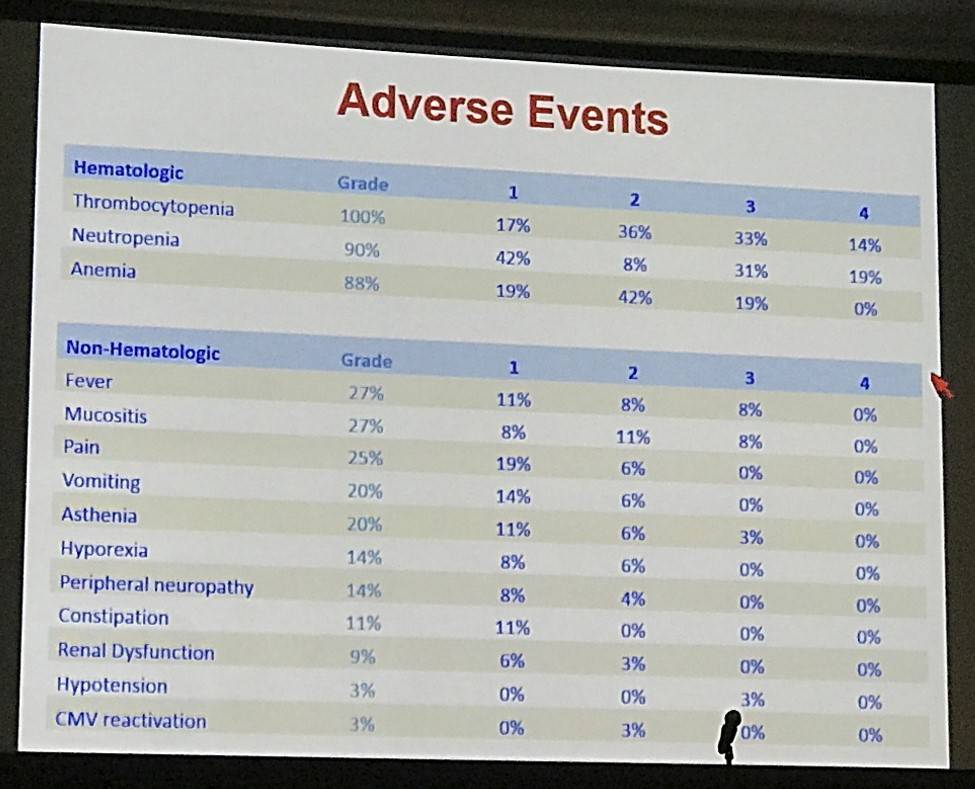
- All patients completed pre-transplant therapy; there were 22 SAEs reported in 15 patients and 2 deaths (non-neutropenic abdominal sepsis and pulmonary embolism)
- Grade 3–4 hematologic AEs were reported in 22 patients: neutropenia (n=18), thrombocytopenia (n=12) and anemia (n=5)
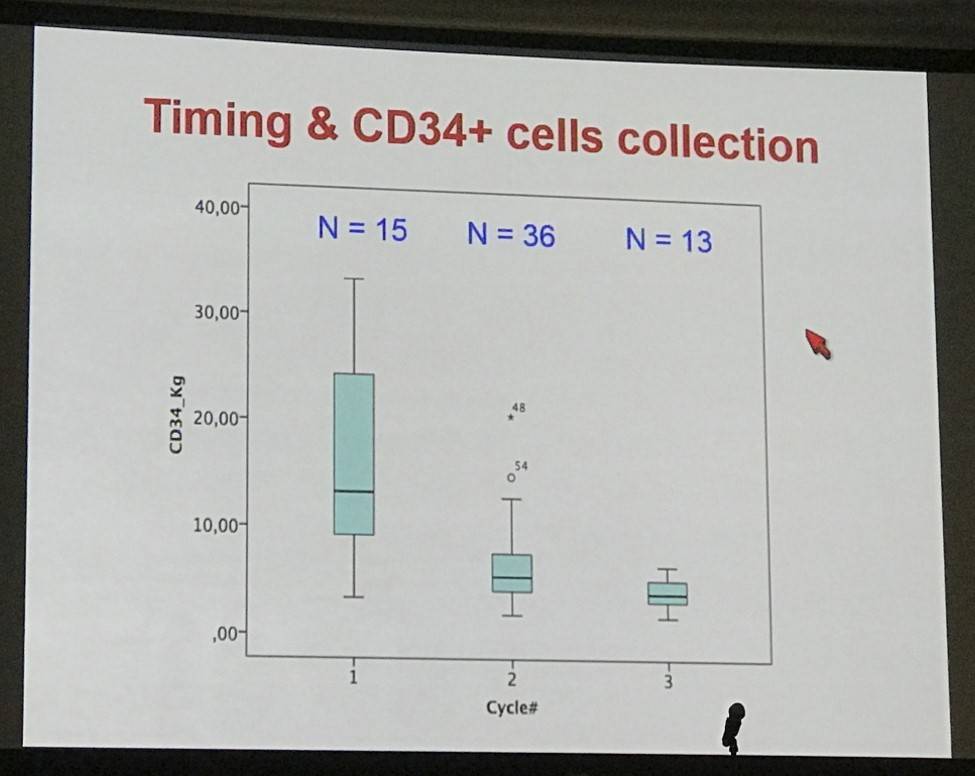
- All but 3 patients underwent stem cell mobilization after the first (n=15), second (n=36) or third (n=12) cycles using subcutaneous G-CSF 5mcg/kg/12 h for 5 days; all patients collected >2.10e6/kg peripheral blood CD34+ cells (median 5.75, range 2.12–33.4)
- Number of harvesting procedures: one n=47 pts, two n=13 pts, three n=2 pts, and four n=1 pt
- Transplantation in 61 patients, data available for 47
- One patient died at day +110 due to pneumonia
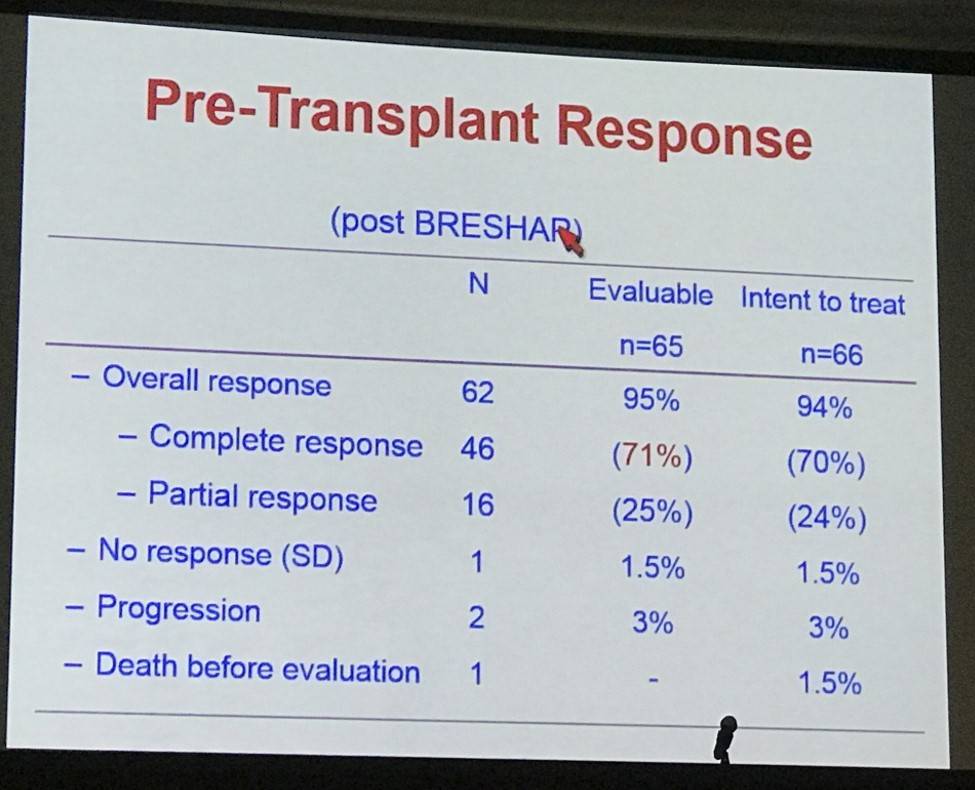
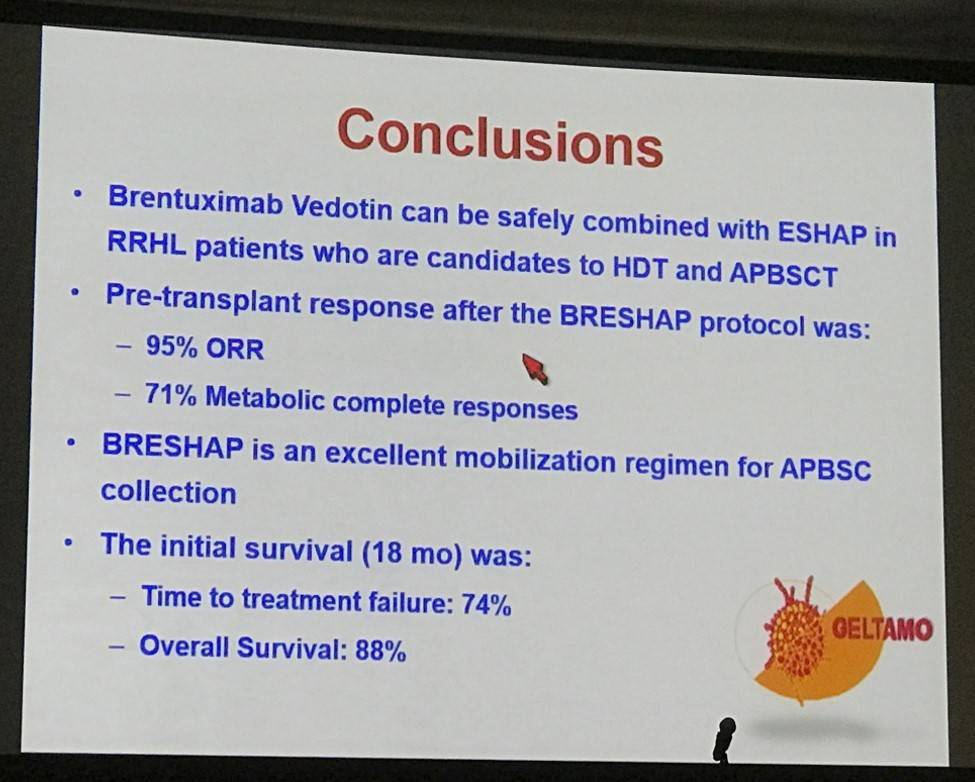
- Overall pre-transplant response in evaluable patients = 95%, CR = 71%, PR = 25%
- Of the evaluable patients, 37 (80%) were in metabolic CR after transplant and 3 (7%) in PR
- Non-responders = 6 patients (13%), these went out of trial
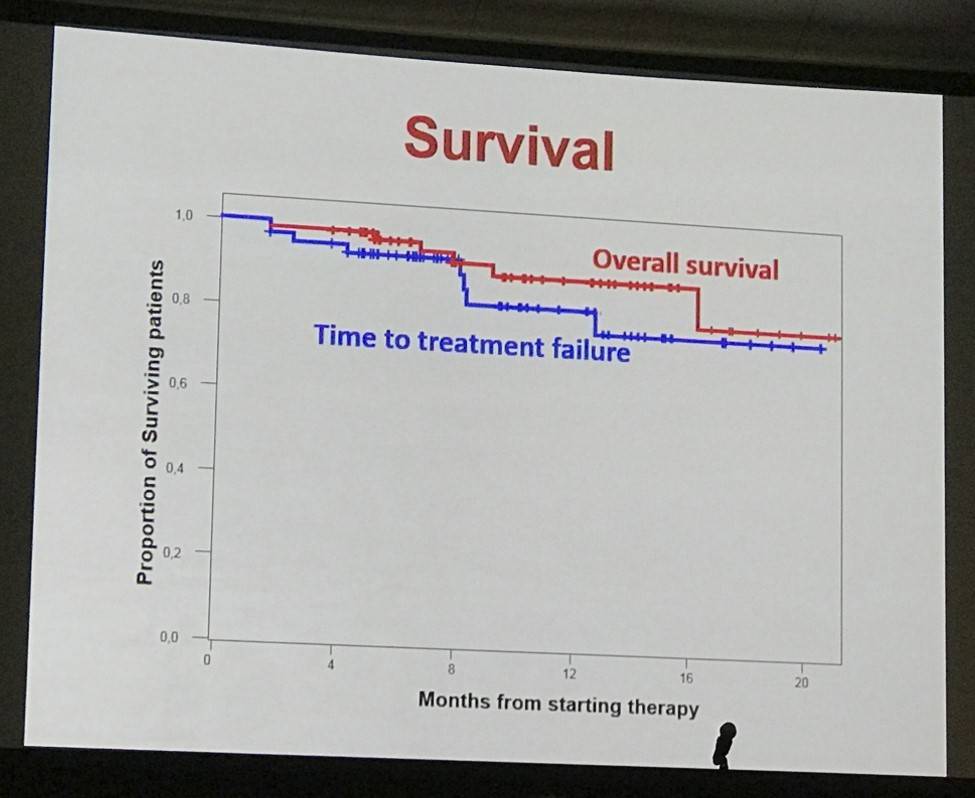
- Median follow-up of 11 months, 7 patients progressed, projected 1-year PFS = 87%
- Three patients died due to progression, total deaths to date = 6
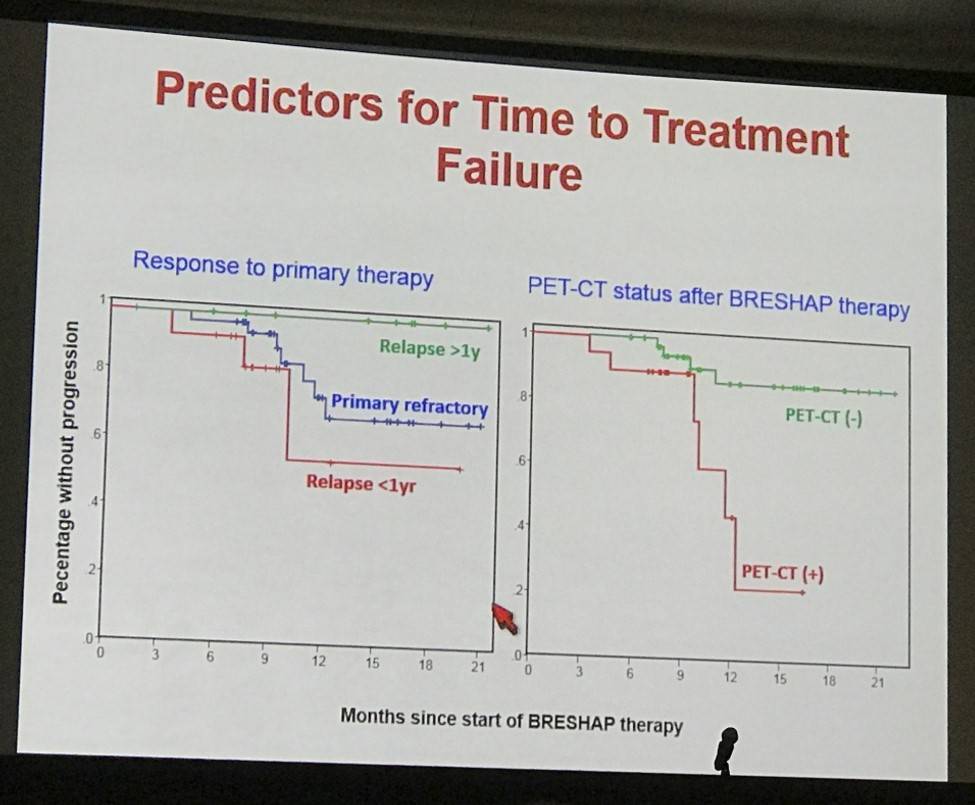
The presentation concluded by stating that brentuximab vedotin plus ESHAP is a highly effective regimen to induce remission before transplant in R/R HL patients. Adding brentuximab vedotin did not negatively affect harvesting of PBSCs and did not increase toxicity of pre- and post-transplant periods.
Abstract:
Introduction: 30% of Hodgkin Lymphoma (HL) patients are refractory or relapse (RR) after first line therapy. Salvage chemotherapy followed by high-dose chemotherapy and with Autologous Peripheral Blood Stem Cell Transplantation (APBSCT) can cure many patients, but those who are transplanted with active disease detectable by PET-CT have a very poor prognosis. Therefore, the current challenge in HL is to improve the results of the pre-transplant chemotherapy. We and others have demonstrated that the addition of Brentuximab Vedotin (BV) to chemotherapy can produce very good results.
Objectives: We conducted a phase II trial to assess response rate with combined Brentuximab vedotin and ESHAP chemotherapy [BRESHAP] as 2nd line therapy for RRHL prior to APBSCT (ClinicalTrials.gov #NCT02243436).
Methods: Primary efficacy endpoint was the proportion of complete responses (CR) pre-APBSCT. A prior phase I step was carried out to establish the appropriate dosis. Final treatment consisted of Brentuximab Vedotin (1.8 mg/m2/day IV, D1), Etoposide (40 mg/m2/day IV, D1-4), Solumedrol (250 mg/day IV, D1-4), High dose AraC (2 g/m2 IV, D5) and cisPlatin (25 mg/m2/day IV, D1-4).
Results: Patients with relapsed or refractory classical HL (cHL) after one prior line of therapy were eligible. 66 patients were included in the trial. There were 35 females and 31 males, with a median age of 36 years (18-66). At inclusion, 40 patients were considered primary refractory, 16 as early relapses (complete remission –CR- shorter than 1 year) and 10 as late relapses. Currently, all patients have completed the pre-transplant therapy. During that period, there were 22 Severe Adverse Events (SAEs) reported in 15 patients: Fever in 13 occasions (neutropenic in seven, and non-neutropenic in six), hypomagnesemia and gastrointestinal alterations (n=2) and pneumothorax, skin lesions, left ventricular function reduction and pulmonary embolism [PE](n=1). There were 2 deaths: non-neutropenic abdominal sepsis and PE. Grade 3-4 hematologic toxicity presented in 22 cases: neutropenia (n=18), thrombocytopenia (n=12), and anemia (n=5). Grade 3-4 extrahematologic adverse events present in ≥5% of cases were non-neutropenic fever (n=8) and hypomagnesemia (n=3). All patients except three underwent stem cell mobilization after the 1st (n=15), 2nd (n=36) or 3rd (n=12) cycle using subcutaneous G-CSF 5 mcg/Kg/12 h. for 5 days. All patients collected >2·10e6/Kg peripheral blood CD34+ cells in all cases (median 5.75, range 2.12-33.4). The number of harvesting procedures was one in 47 patients, two in 13, three in 2 and four in 1. The transplant has been done in 61 patients, with data are available from 47: all engrafted with a median of 9&10 days for neutrophil and platelet recovery, respectively. No major events were registered during transplant period, except for one patient who died at day +110 due to pneumonia. Overall pre-transplant response was 96%, including a 70% and 26% complete and partial remission rates, respectively. Of these forty-seven patients, 37 (80%) were in metabolic CR after transplant and 3 (7%) in PR; six patients were considered as non-responders (13%) and went out of the trial. At a mean follow-up of 11 months, 7 patients have progressed, rendering a projected progression free survival of 87% at one year. Six patients have already died: three due to progression, and the three already mentioned above (PE, abdominal sepsis and pneumonia). With a mean follow-up of 11 months, the projected overall survival was 90% at one year (cause specific, 96%).
Conclusions: BRESHAP is a highly effective regimen for remission induction prior to transplant in patients with refractory or relapsed Hodgkin lymphoma. The addition of BV to the conventional chemotherapy did not resulted in a higher toxicity for the pre- and post-transplant periods and it did not hamper the collection of PBSC.
- Garcia-Sanz R. et al. Brentuximab vedotin plus ESHAP (BRESHAP) is a Highly Effective Combination for Inducing Remission in Refractory and Relapsed Hodgkin Lymphoma Patients Prior to Autologous Stem Cell Transplant: A Trial of the Spanish Group of Lymphoma and Bone Marrow Transplantation (GELTAMO). Oral Abstract #1109: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








