All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Ibrutinib plus palbociclib in patients with previously treated Mantle Cell Lymphoma
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology (ASH) took place in San Diego, CA, on December 3–6, 2016.
On Saturday 3rd December, an oral abstract session was held between 12.00pm and 1.30pm in the “Mantle Cell, Follicular, and other Indolent B-Cell Lymphoma – Clinical Studies: Therapeutic Approaches to Mantle Cell Lymphoma” category. This session was moderated by Anca Prica, MD, from the Princess Margaret Cancer Centre, and Constantine S. Tam, MBBS, from the Peter MacCallum Cancer Centre & St Vincent’s Hospital.
Abstract #150 was presented during this session titled “A phase I trial of ibrutinib plus palbociclib in patients with previously treated Mantle Cell Lymphoma” by Peter Martin, MD, from Weill Cornell Medical College, New York, and colleagues.
This group conducted a phase I trial (NCT02159755) aimed at evaluating the safety and activity of palbociclib in combination with ibrutinib in patients with previously treated Mantle Cell Lymphoma (MCL). The primary endpoint of this study was to estimate the Maximum Tolerated Dose (MTD) of the combination. The key highlights are:
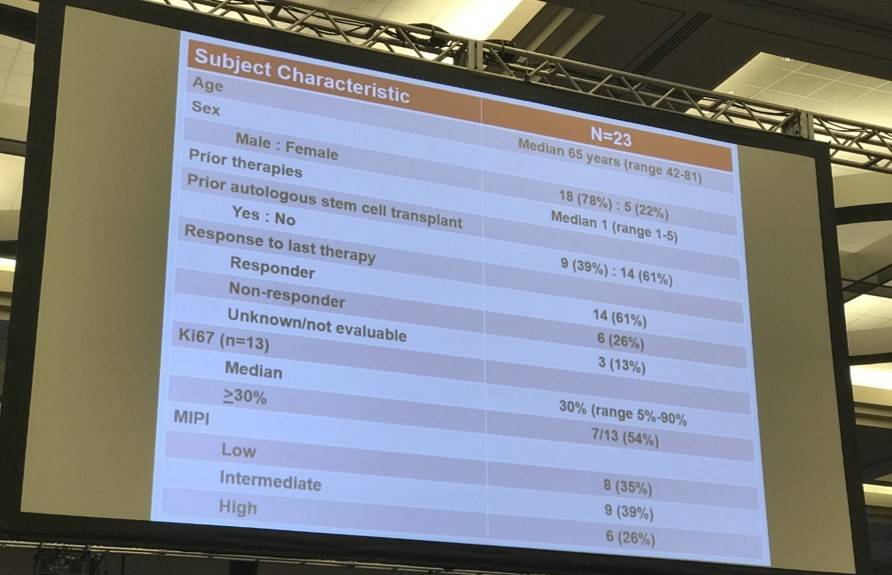
- 23 patients (median age = 65 years) were enrolled; 18 Males, 5 females
- Median number of prior therapies = 1
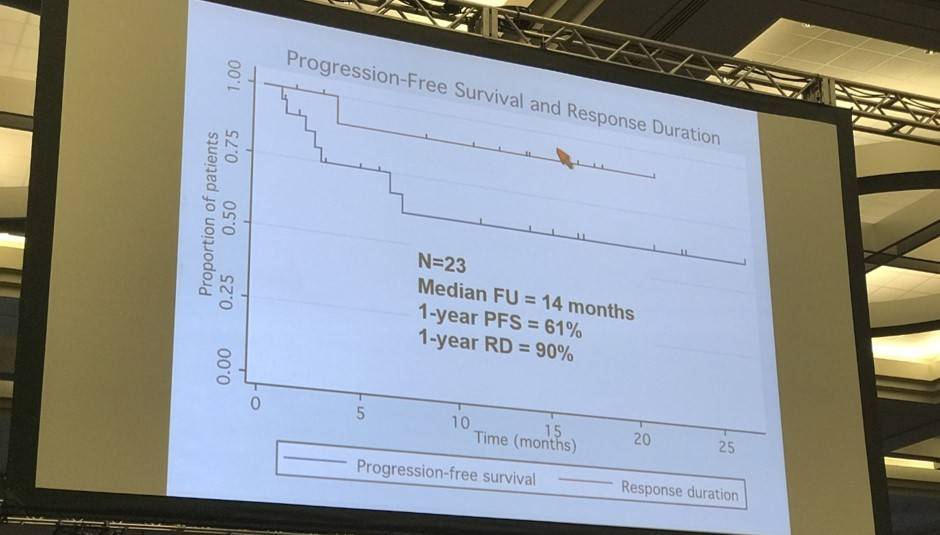
- At median follow up of 14 months, 1-year Progression Free Survival (PFS) = 61%
- 1-year Response Duration (RD) = 90%
- Dose Limiting Toxicity (DLT) occurred in 3 patients; including grade 4 thrombocytopenia (1) and grade 3 rash (2)
- Grade 3–4 hematological toxicities occurred; thrombocytopenia (26%), neutropenia (30%) and lymphopenia (17%)
- 12 patients responded to treatment and 8 achieved a Complete Response (CR)
Peter Martin concluded the presentation by highlighting that ibrutinib in combination with palbociclib is well tolerated and active in MCL patients. The MTD was 560mg ibrutinib plus 100mg palbociclib. A multi-center phase II trial is planned.

Abstract
Background
Single-agent ibrutinib confers a response rate of 77%, including a complete response (CR) rate of 19% in patients with previously treated mantle cell lymphoma (MCL); however, with a median progression-free survival (PFS) of 14.6 months and 1-year response duration (RD) rate of 69%, nearly half of all patients experience treatment failure during the first year. We previously demonstrated that prolonged early G1 cell cycle arrest induced by the oral, specific CDK4/6 inhibitor palbociclib can overcome ibrutinib resistance in primary human samples and MCL cell lines with wild-type BTK (Chiron et al. Cancer Discovery 2014). We conducted a phase I trial to evaluate the safety and preliminary activity of palbociclib plus ibrutinib in patients with previously treated mantle cell lymphoma.
Methods
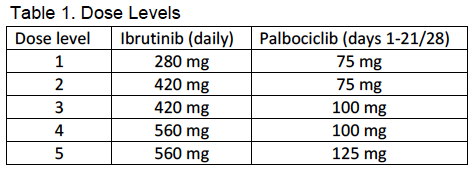
Adult patients who were ibrutinib and CDK4/6 inhibitor-naïve who had previously treated MCL were eligible to participate. The primary objective was to estimate the maximum tolerated dose of the combination. Consenting patients were enrolled to one of five dose levels, shown in Table 1. Patients were treated in 28 day cycles, with ibrutinib administered daily and palbociclib administered on days 1-21. (Table 1). Patients could continue to receive study treatment until progression, unacceptable toxicity, or withdrawal of consent. Doses were escalated according to a standard phase I 3+3 design. Patients were evaluated for efficacy at the end of cycles 3 and 6, and every 6 cycles thereafter. All CRs, as documented by CT, required confirmation by PET/CT; bone marrow biopsy and endoscopy were also required in patients with known marrow or GI tract involvement, respectively. Additional objectives included pharmacokinetics and evaluation of pretreatment samples for biomarkers of response or resistance.
Results
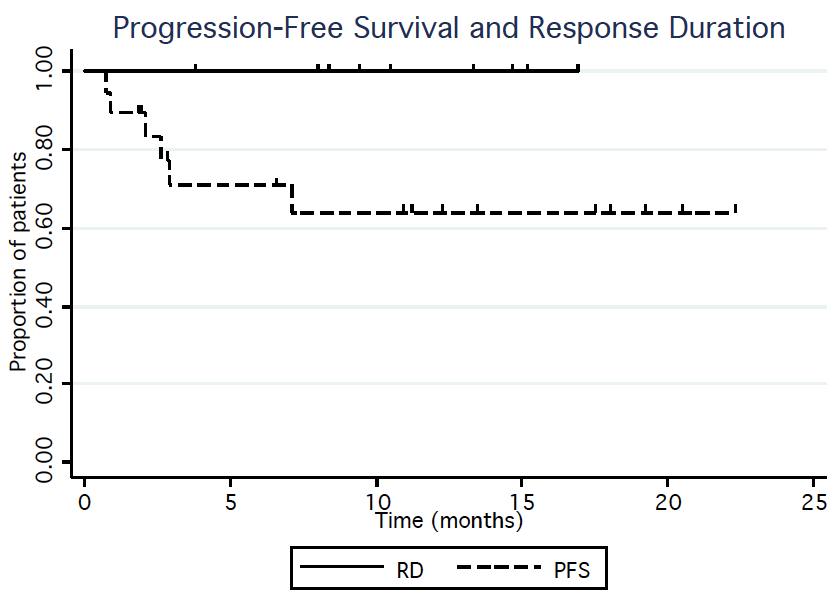
From August 2014 to June 2016 a total of 20 patients (15 males, 5 females) were enrolled (DL1 n=3, DL2 n=3, DL3 n=6, DL4 n=3, DL5 n=5). The patients' MIPI risk distribution were 7 low, 7 intermediate, and 6 high. The median number of prior therapies was 1 (range 1-5). Six patients were refractory to their last prior therapy. Three patients experienced dose limiting toxicity: One patient treated at DL3 experienced grade 4 thrombocytopenia lasting more than 7 days, and grade 3 rash was seen in two patients at DL5. Grade 3-4 hematological toxicity included thrombocytopenia (28%), neutropenia (22%), and lymphopenia (17%). Grade 3-4 non-hematological toxicity regardless of attribution included one patient with each of the following: lung infection, ALT/AST increase, encephalitis, hyponatremia, sinus tachycardia, pneumonitis. Grade 1-2 adverse events related to treatment and occurring in at least 2 patients included the following: diarrhea (50%), fatigue (44%), rash (39%), bruising (17%), nausea (17%), fever (11%), dyspepsia (11%), and myalgia (11%). Other than the two patients that experienced grade 3 rash at DL5, no patients have required dose reductions; 6 patients required dose interruptions. Thirteen subjects continue on study therapy. The reasons for stopping treatment were disease progression (n=4), adverse event (elevated liver enzymes, n=1; and prolonged cytopenias, n=1), and allogeneic stem cell transplantation (n=1). Of the 18 patients that have had at least one response evaluation to date, 12 (67%) patients responded to treatment and 8 (44%) achieved a CR. The median time to CR was 3 cycles and no responding patients have progressed on study. With a median follow up of 11 months, the estimated 1-year PFS and RD are 68% and 100%, respectively (Figure 1).
Conclusions
The mechanism-based combination of ibrutinib plus palbociclib is well tolerated and active. Toxicity is primarily related to myelosuppression of grade 1-2 severity, although grade 3 rash was observed at the highest doses evaluated. In this small group of patients, the combination produced responses at all dose levels, with a CR rate of 44% and a median time to CR of 3 months. No responding patients have progressed to date. These preliminary CR, PFS, and RD rates appear better than those reported in other studies of single-agent ibrutinib although the numbers of patients was very small. A phase II multi-center clinical trial to evaluate time to progression is planned. Biomarker studies to evaluate mechanisms of primary resistance are ongoing.
- Martin P. et al. A Phase I Trial of Ibrutinib Plus Palbociclib in Patients with Previously Treated Mantle Cell Lymphoma. 2016 December 3; Oral Abstract #150: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








