All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Idelalisib + BR significantly improves OS compared to BR alone: updated OS results of Phase-III trial in R/R CLL patients
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology (ASH) took place on 3–6 December 2016 in San Diego, CA. On Saturday 3rd December, an oral abstract session was held between 4:00pm and 5:30pm in the “CLL: Therapy, Excluding Transplantation Program” category. This session was moderated by Tait D. Shanafelt, MD, of the Mayo Clinic, and John F. Seymour, MB BS PhD FRACP, of the Peter MacCallum Cancer Centre and Royal Melbourne Hospital.
Abstract #231 was presented during this session, titled “Updated Analysis of Overall Survival in Randomized Phase-III Study of Idelalisib in Combination with Bendamustine and Rituximab in Patients with Relapsed/Refractory CLL” by Andrew D. Zelenetz, MD, PhD, of the Memorial Sloan Kettering Cancer Center, New York, and colleagues.
This abstract presented updated data on Overall Survival (OS) of a phase III, double-blind, randomized, placebo-controlled study (NCT01569295) aimed at evaluating the safety and efficacy of idelalisib plus bendamustine and rituximab for patients with previously treated CLL.
The primary end-point of this study was Progression Free Survival (PFS) based on Independent Review Committee, and the secondary end-point was OS. Four-hundred and sixteen patients were enrolled between June 2012 and August 2014. At the cut-off date of May 2016, and a median follow-up of 21 months:
- All patients completed study treatment with bendamustine rituximab; 65 patients remain on treatment (idelalisib monotherapy = 64, placebo = 1)
- Overall, by ITT and IRC, 260/416 patients met the primary endpoint of PD or death; idelalisib n=95, placebo n=165
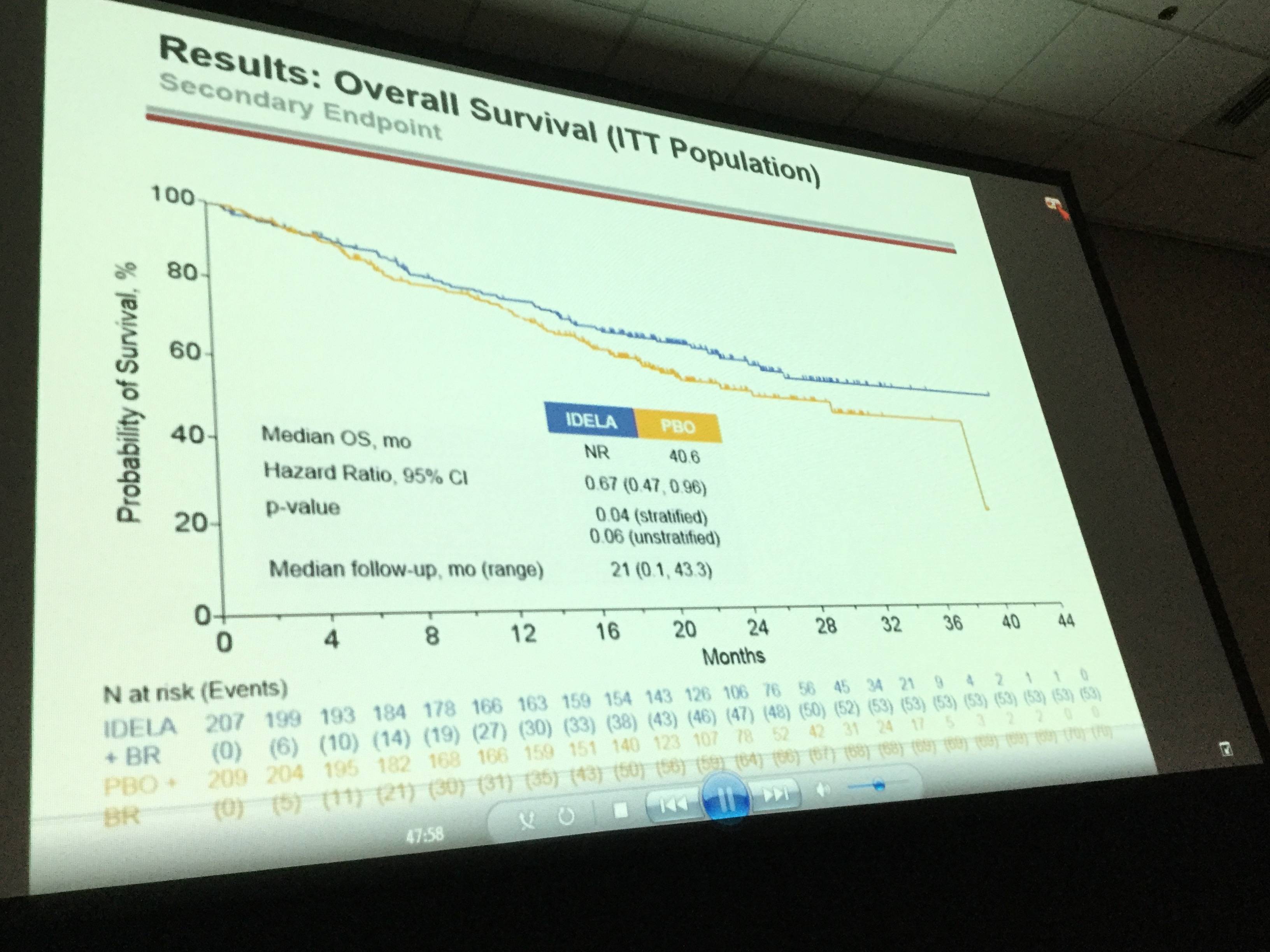
- Median OS of idelalisib+BR versus BR+placebo was not reached versus 6 months (HR = 0.67; p value 0.036; 95% CI 0.47, 0.96)
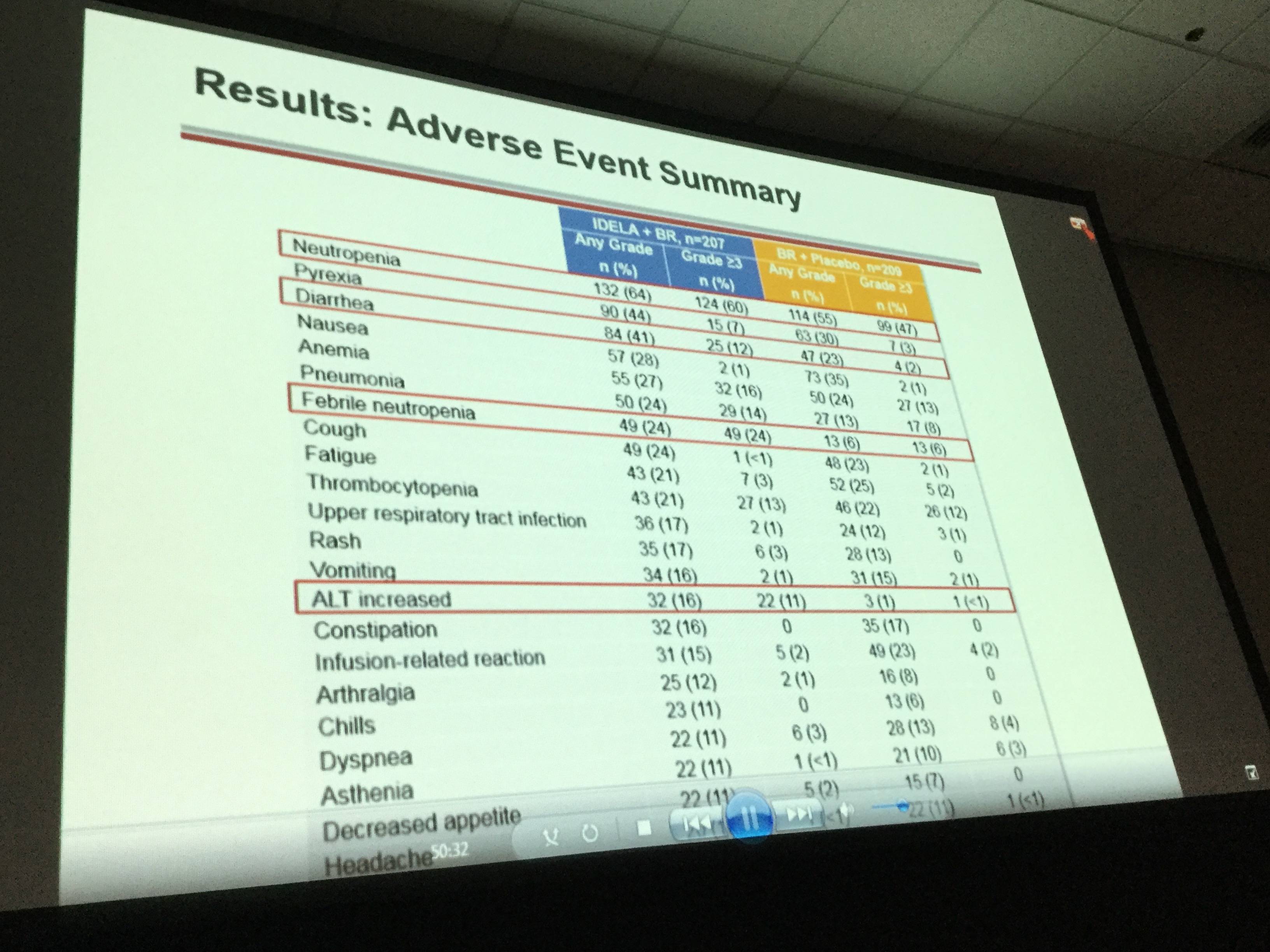
- SAEs were reported in 147 (71%) of idelalisib arm patients and in 94 (5%) of placebo arm patients
- In the idelalisib and placebo arms, the most frequent SAEs by system organ class were infections (41% vs 23%) and, by MEDRA-preferred terms, febrile neutropenia (21% vs 5%) and pneumonia (17% vs 8%), respectively
- Pneumocystis jirovecii pneumonia was reported in 5 idelalisib arm patients compared to 0 patients in the placebo arm. Cytomegalovirus infections occurred in 13 idelalisib arm patients compared to 3 placebo arm patients
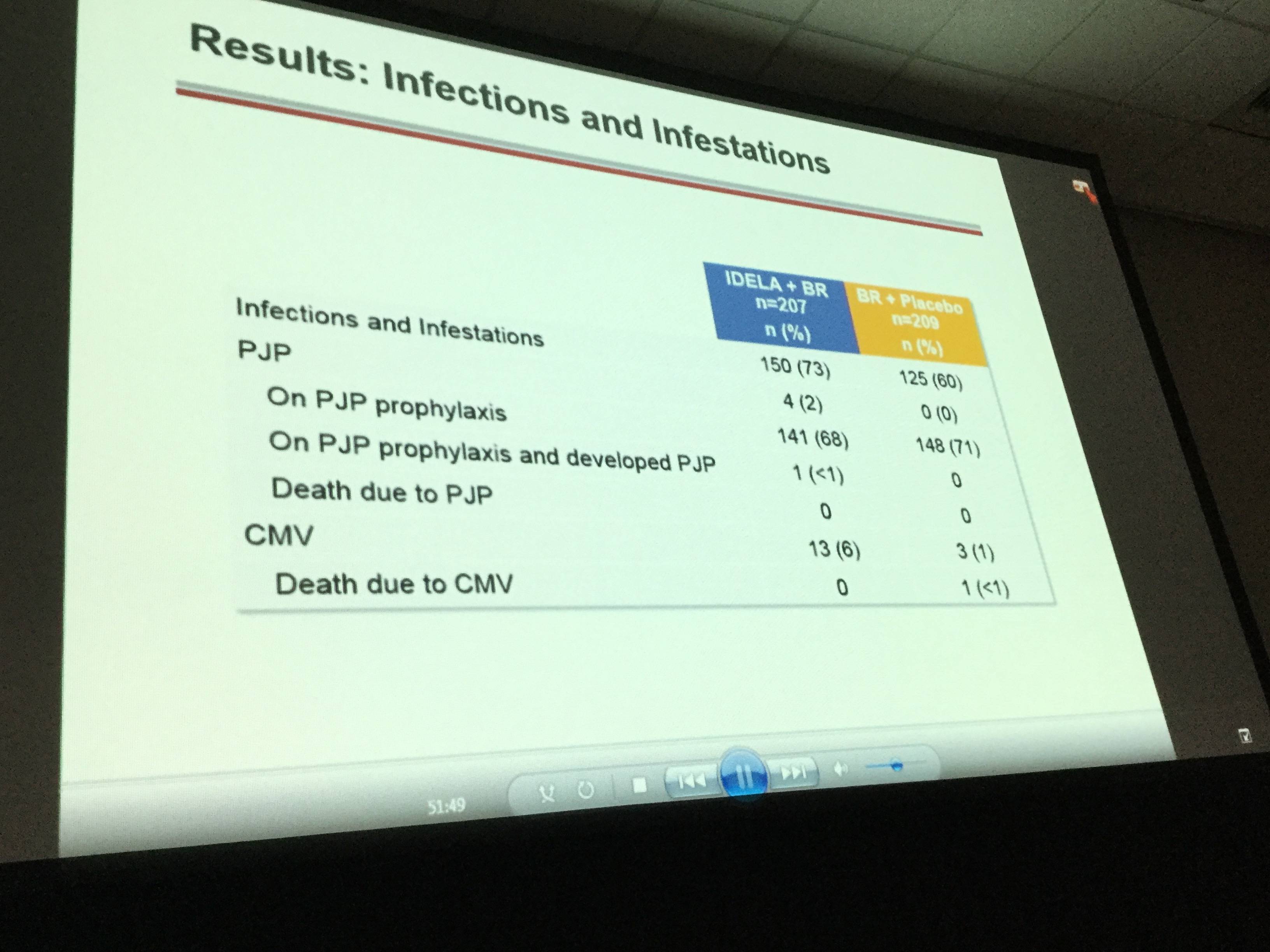
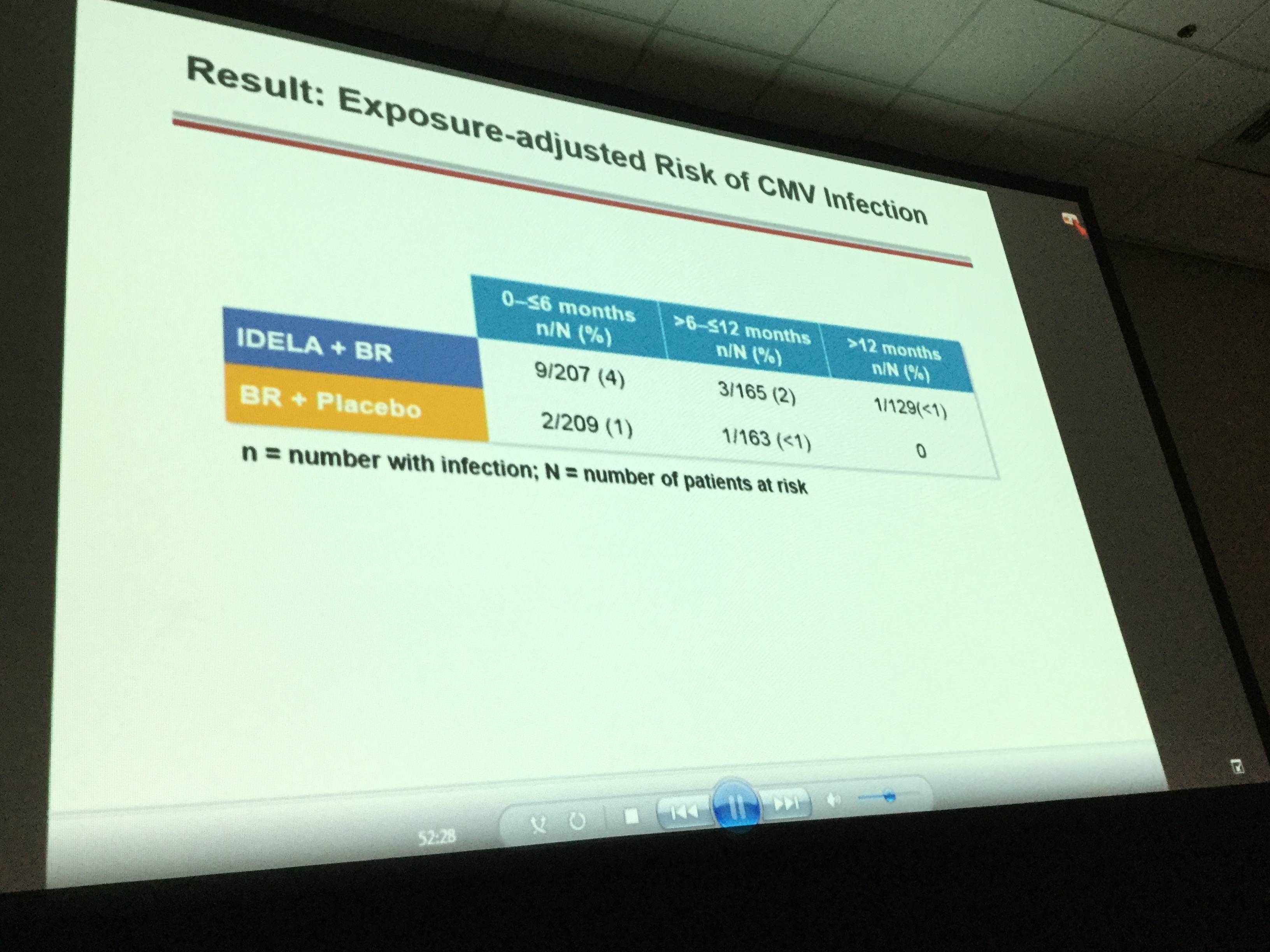
It was concluded that superior OS in R/R CLL is achieved when treated with idelalisib plus bendamustine and rituximab compared to bendamustine and rituximab alone. However, SAEs and opportunistic infections were more frequent in the idelalisib-containing regimen compared to the placebo arm. In future, idelalisib-containing regimens could be improved by implementing adequate monitoring of Pneumocystis jirovecii pneumonia and cytomegalovirus infection. Idelalisib + bendamustine and rituximab provides a new, alternative treatment option for R/R CLL patients.
Abstract
Introduction: Therapies able to improve overall survival in patients with relapsed/refractory (RR) CLL are needed. We have previously reported that idelalisib (IDELA), a selective PI3K delta inhibitor, administered in combination with bendamustine/rituximab (BR) improves progression-free survival compared with BR alone after a medianfollowup of 12 months. This study (NCT01569295) was unblinded by the independent data monitoring committee at first interim analysis for efficacy. We now present updated data on overall survival (OS).
Methods: Between June 2012 and August 2014, 416 patients (pts) with RR CLL were enrolled in the study across 19 countries. The current analysis data cutoff date of May 2016 represents a median follow-up of 21 months. Progression-free survival based on independent review committee assessment was the primary endpoint of this study, with OS as a secondary endpoint. All pts had completed study treatment with BR. Key eligibility criteria included pts with RR CLL requiring therapy, having received previous purine analog or bendamustine (ineligible if refractory to bendamustine); and anti-CD20 antibody; relapsing or progressing within 36 months of the completion of the last therapy. Patients were randomized to BR for 6 cycles Q 28 days (B = 70 mg/m2 D1, D2 of each cycle; R = 375 mg/m2 C1 and 500 mg/m2 C2-6) and IDELA 150 mg BID or placebo (administered until IRC-confirmed PD), death, intolerable toxicity, or withdrawal of consent. Stratification was based on presence/absence of del(17p) and/or p53 mutation (mut), immunoglobulin heavy chain variable region (IGHV) mutated/unmutated (analysis performed by a central lab), and disease status refractory (CLL progression <6 months from completion of prior therapy) vs relapsed (CLL progression ≥6 months from completion of prior therapy). Crossover was not permitted at the time of PD or unblinding.
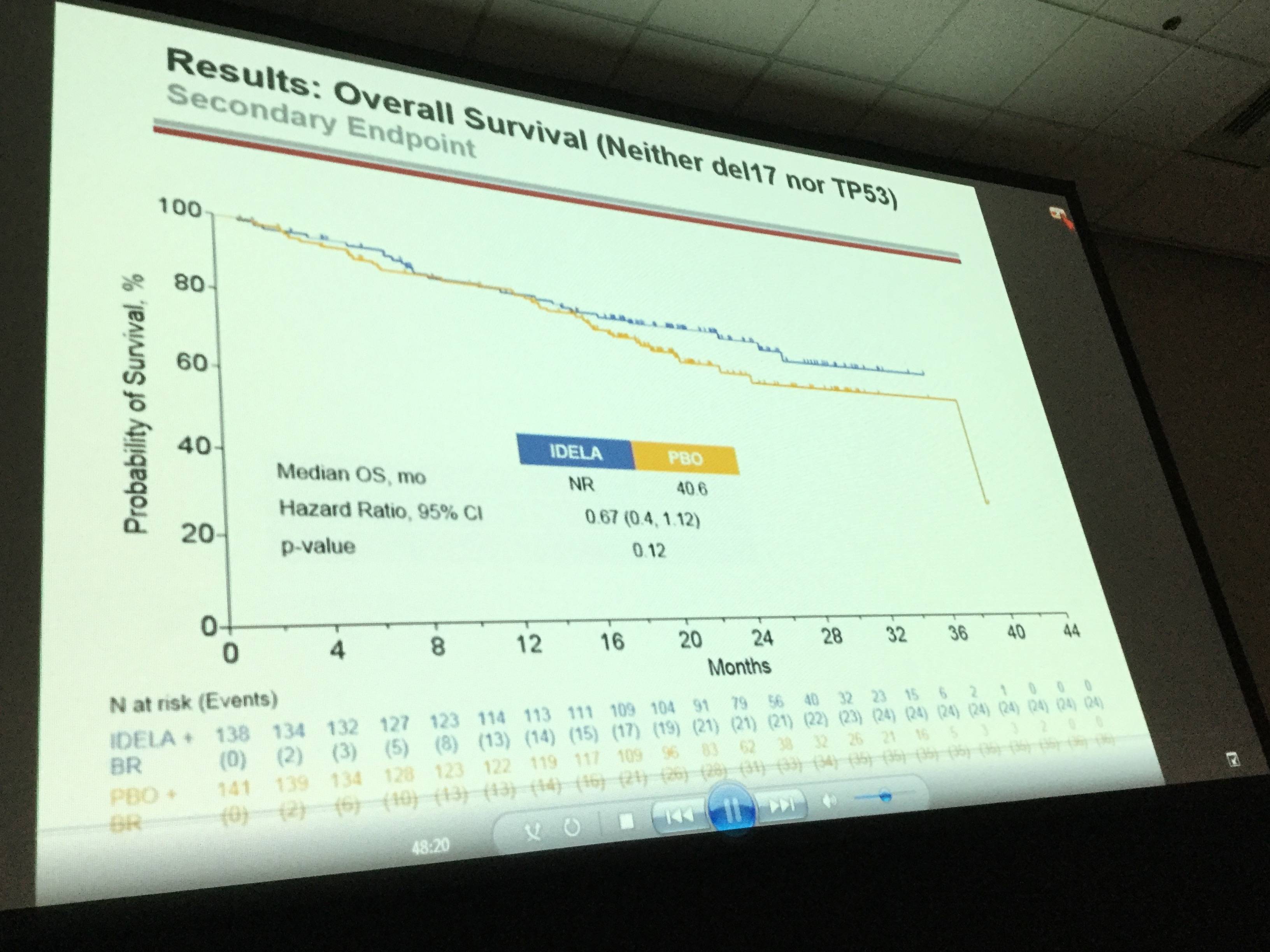
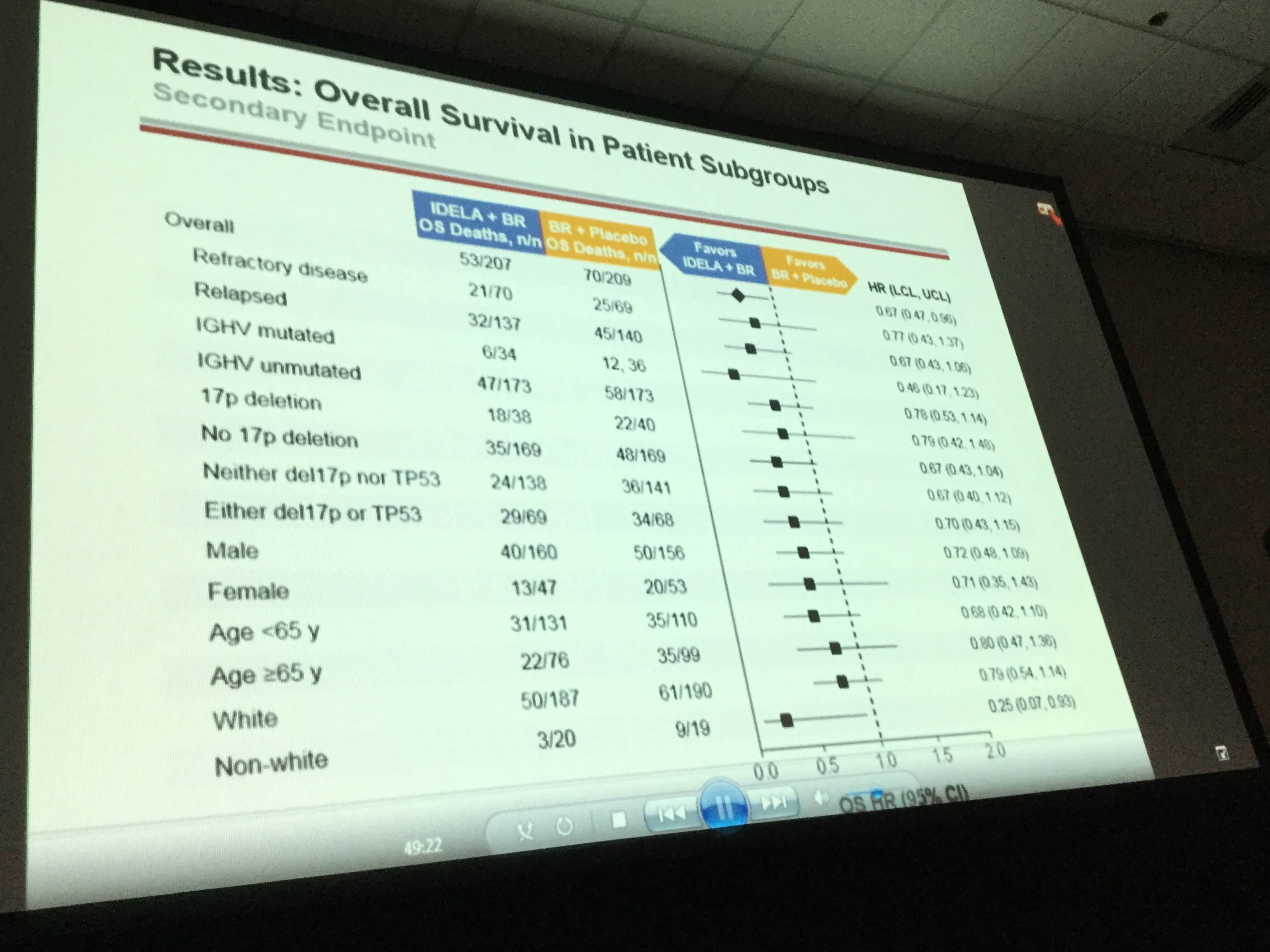
Results: The ITT population reflects 207/209 pts in the IDELA + BR/BR + placebo arm: 76% male; 42% ≥65 years; Rai stage III/IV 46%; median time since completion of last prior therapy 16 months; pts with high-risk features (del[17p]/p53mut 32.9%, unmutated IGHV 83.2%, refractory 29.8%); median number of prior therapies: 2 (range 1–13); and median follow-up 21 months. All pts have completed study treatment with BR. A total of 65 pts remain on study treatment: 64 on IDELA monotherapy and 1 pt on placebo. Overall by ITT and IRC, 260/416 pts (IDELA/placebo 95/165) have met the primary endpoint of PD or death. Median OS (mo) of IDELA + BR vs BR + placebo was not reached vs 41 (HR = 0.67; p value 0.036; 95% CI 0.47, 0.96) (Figure 1). The safety findings were similar to what we previously reported: Serious AEs occurred in 147 (71%)/94 (5%) IDELA/placebo arms, respectively. The commonly occurring SAEs by system organ class were infections and infestations (41%/23%) and by MEDRA-preferred terms febrile neutropenia 43 (21%)/10 (5%) and pneumonia 35 (17%)/16 (8%) in the IDELA/placebo arms respectively. The total number of pts with opportunistic infections (Pneumocystis jiroveciipneumonia [PJP]/cytomegalovirus [CMV]) in the IDELA arm was 5/13 vs 0/3 in the placebo arm.
Conclusion: IDELA in combination with BR is superior to BR alone with regard to OS in RR CLL. The improvement in OS was observed across risk categories. Opportunistic infections (PJP, CMV) and SAEs were more frequent in the IDELA vs placebo arm. Results of IDELA-containing regimens may be further improved with implementation of adequate PJP prophylaxis and CMV monitoring measures. This regimen represents an important new option for pts with RR CLL.
- Zelenetz A.D. et al. Updated Analysis of Overall Survival in Randomized Phase III Study of Idelalisib in Combination with Bendamustine and Rituximab in Patients with Relapsed/Refractory CLL. Oral Abstract #231: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








