All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Support for Relapsed or Refractory Primary CNS Lymphoma – a Prospective Multicenter Trial by the German Cooperative PCNSL Study Group
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, and on December 5th, Benjamin Kasenda, MD, PhD, from the Haematology/Oncology Center at Klinikum-Stuttgart, Germany, presented data from a prospective single-arm, multi-center, phase-II trial conducted by the German Cooperative PCNSL Study Group.
Highlights:
- Treatment: 2 courses rituximab, thiotepa, high-dose cytarabine followed by HCT-ASCT regardless of response. No CR following HCT-ASCT underwent whole-brain radiotherapy (WBRT)
- 39 pts from 12 centers, <66 years old (median=57yr) who failed prior HD-MTX therapy. Of these, 28 were relapsed, 8 refractory
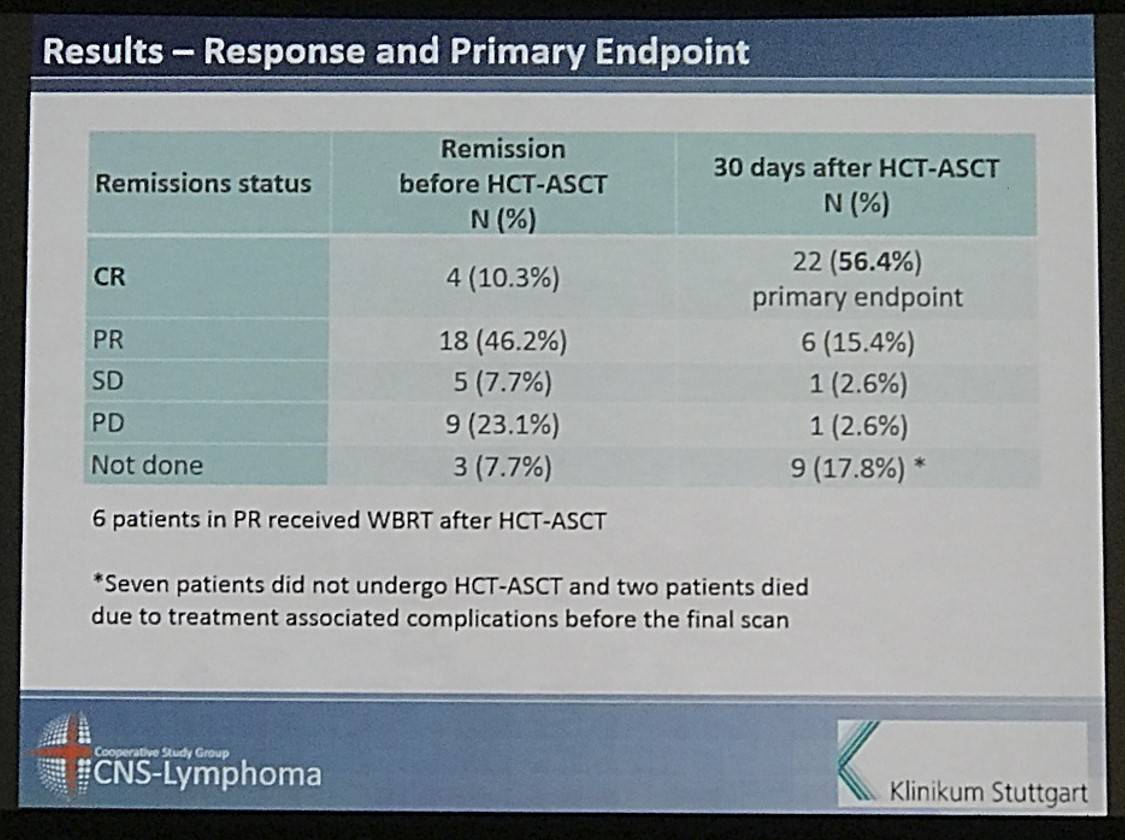
- 4% responded to induction before HCT-ASCT (4CR, 18PR)
- 1% pts underwent HCT-ASCT (32pts), after this CR=56.4% (22pts), PR=15.4% (6pts)
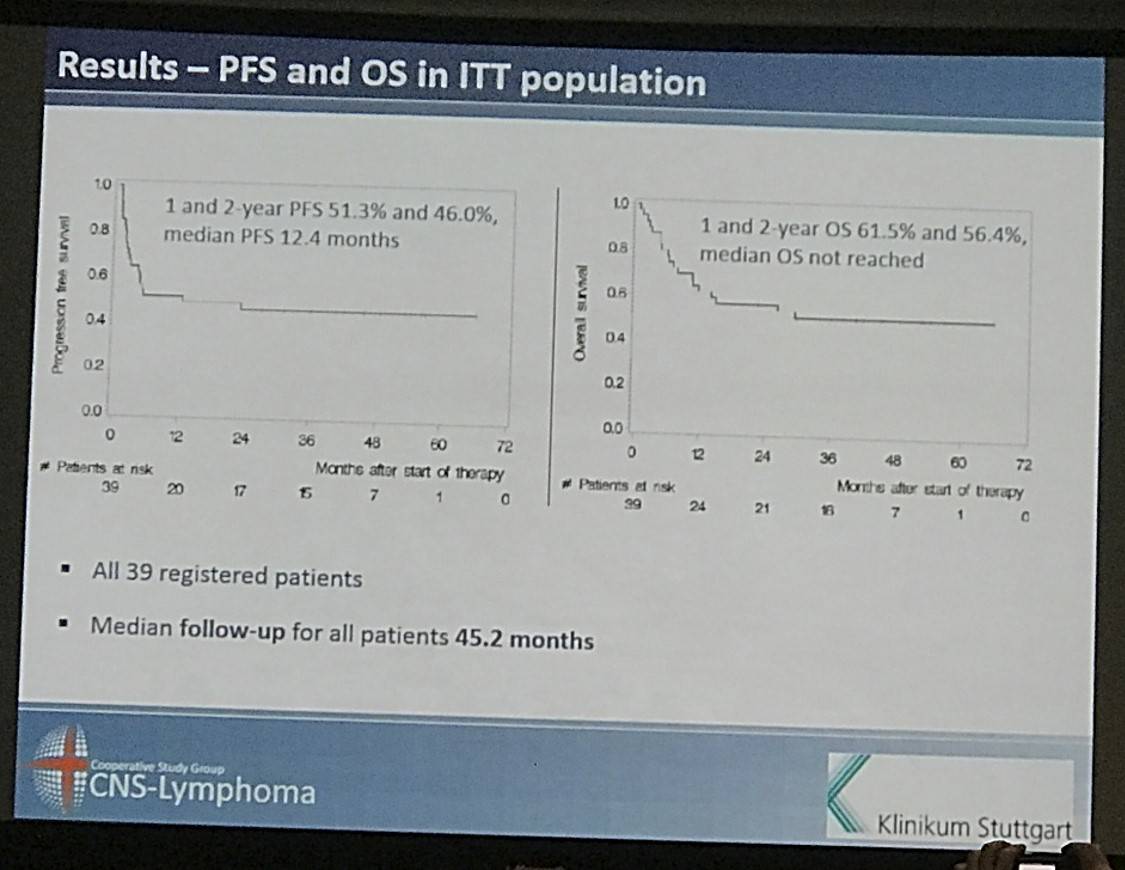
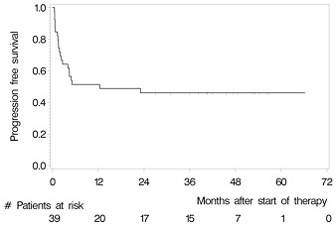
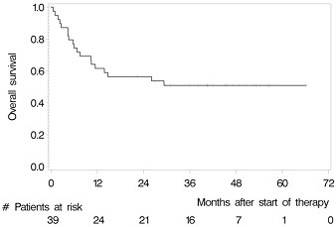
- Overall 2 year OS = 56.4% and PFS = 46%
- Following HCT-ASCT 1yr PFS = 62.5%. 2yr PFS = 56.1%
- In the ITT population, 1yr PFS = 51.3%. 2yr PFS = 46.0%
- 1yr and 2yr OS in the ITT population are 61.5% and 56.4%
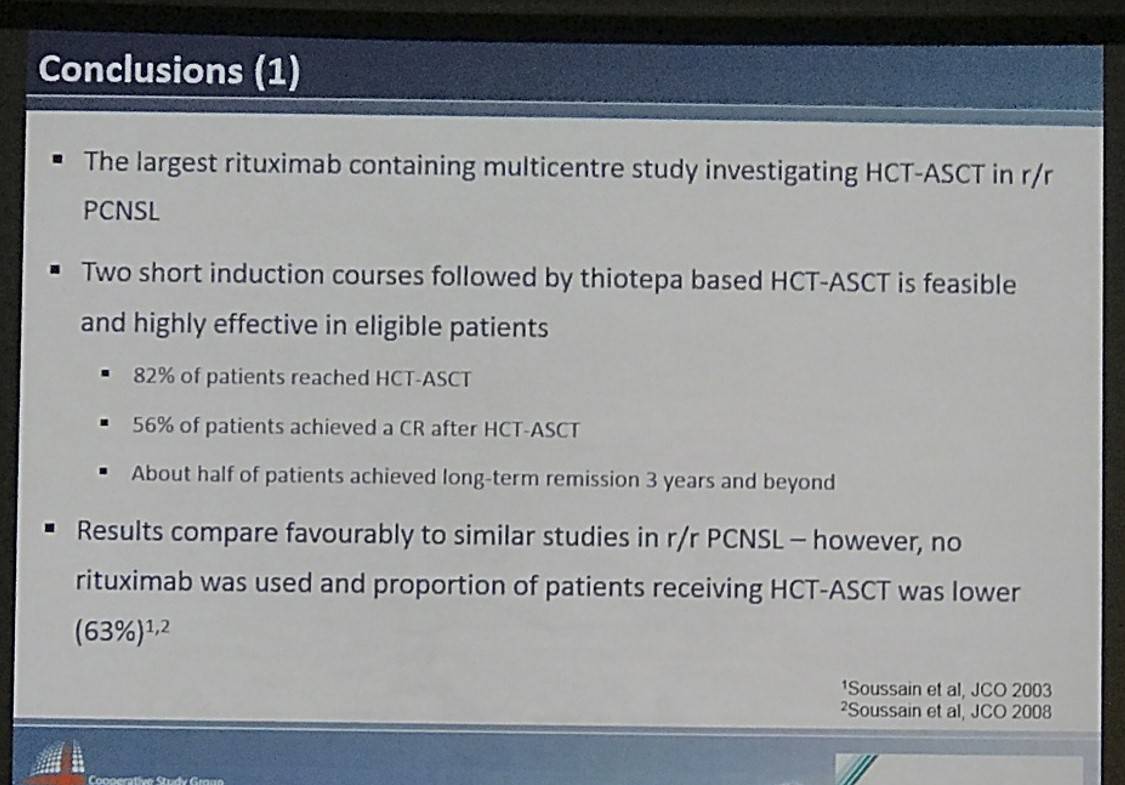
In conclusion it was stated that short-induction therapy, like that outlined here, followed by HCT-ASCT is effective in R/R PCNSL patients having failed prior HD-MTC therapy, but comparative studies will need to be published to further analyze the role of HCT-ASCT.

Abstract:
Purpose: To investigate safety and efficacy of high-dose chemotherapy followed by autologous stem cell transplantation (HCT-ASCT) in patients with relapsed or refractory primary CNS lymphoma (PCNSL).
Patients and methods: We conducted a single-arm multicentre phase 2 study for immunocompetent patients (<66 years of age) with PCNSL failing prior HD-MTX based chemotherapy. Induction treatment consisted of 2 courses of rituximab (rituximab 375mg/m2), high-dose cytarabine (2 x 3g/m2) and thiotepa (40mg/m2) with collection of autologous stem cells in between. Conditioning treatment for HCT-ASCT consisted of rituximab 375mg/m2, carmustine 400mg/m2 and thiotepa (4 x 5mg/kg). Patients commenced HCT-ASCT irrespective of response status after induction. Only patients not achieving complete remission (CR) after HCT-ASCT received whole brain radiotherapy (WBRT). The primary endpoint was CR after HCT-ASCT by intention-to-treat (ITT). Secondary endpoints included safety, progression free survival (PFS, time to progression or death) and overall survival (OS, time to death due to any cause).
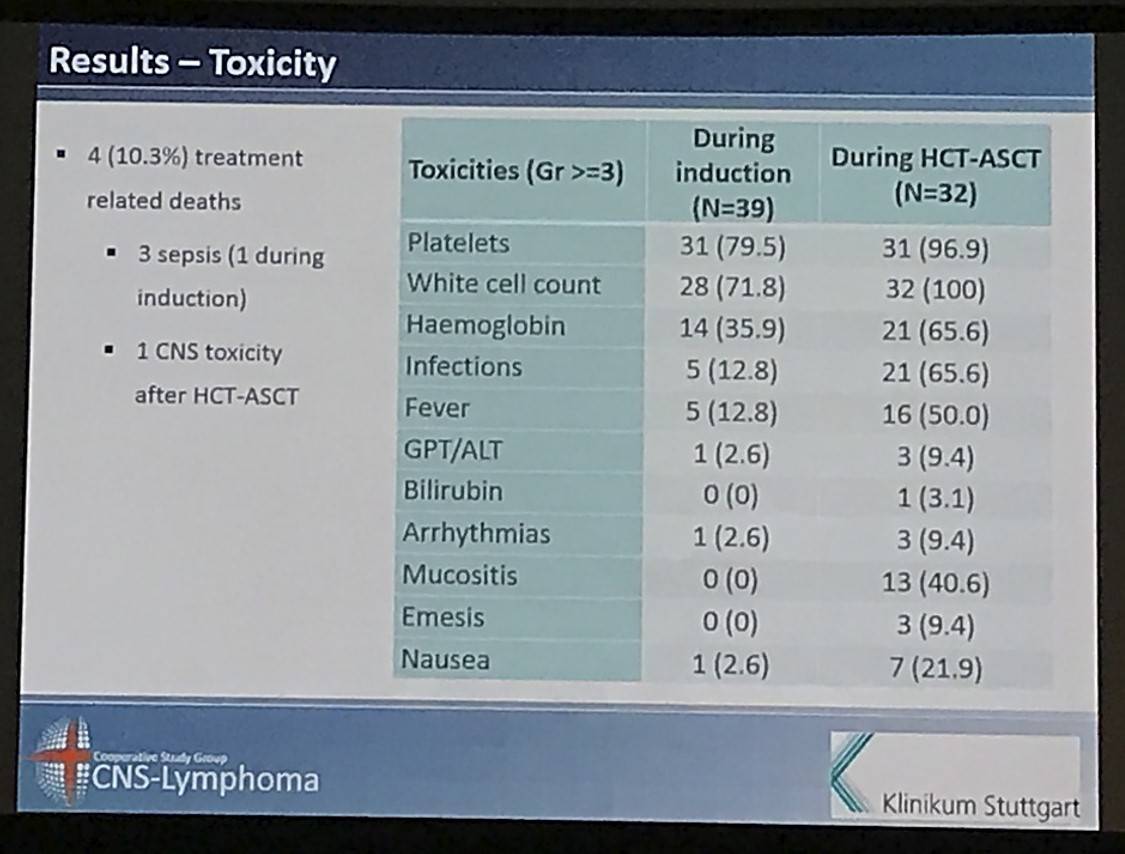
Results: Between May 2007 and July 2012, we enrolled 39 patients from 12 German centres. The median age and Karnofsky performance score was 57 years (range 37 to 65) and 90% (range 60% to 100%), respectively. 28 (71.8%) patients had relapsed and 8 (28.2%) refractory disease. 22 (56.4%) patients responded to induction (4 CR, 18 partial remissions [PR]) and 32 (82.1%) patients commenced HCT-ASCT. 22 patients (56.4%, 95% CI 39.6% to 72.2%) achieved CR after HCT-ASCT, 6 (15.4%) achieved PR, and 1 (2.6%) had stable disease. In 9 (17.8%) patients the final scan was not done, because 7 (18.0%) did not undergo HCT-ASCT and 2 died (5.1%) during HCT-ASCT procedure. After a median follow-up of 45.2 months, the respective 2-year PFS and OS rates were 46.0% (95% CI 30.3% to 61.7%, median PFS 12.4 months, Figure 1) and 56.4% (95% CI 40.8% to 72.0%); median OS not reached (Figure 2). The non-relapse mortality rate was 10.3% (95% CI 4.1% to 26.0%) at 1 year without any further increase afterwards. In the subset of 32 patients who received HCT-ASCT, 14 (56.3%) experienced progression or died translating into 1 and 2-year PFS rates (calculated from date of HCT-ASCT) of 62.5% (95% CI 45.7% to 79.3%) and 56.1% (95% CI 38.8% to 73.3%) with no further decrease afterwards. Main grade 3 or higher toxicities were hematological as expected. We recorded four (10.3%) treatment-related deaths, 2 during induction and 2 during HCT-ASCT.
Conclusions: In eligible PCNSL patients failing HD-MTX based chemotherapy, a short induction with high-dose cytarabine and thiotepa followed by HCT-ASCT is an effective treatment option. Our data provide a reliable benchmark for future comparative studies needed to further scrutinize the role of HCT-ASCT in the salvage setting for PCNSL.
- Kasenda B. et al. High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Support for Relapsed or Refractory Primary CNS Lymphoma – a Prospective Multicentre Trial By the German Cooperative PCNSL Study Group. 2016 December 5; Oral Abstract #781: ASH 58th Annual Meeting and Exposition, San Diego, CA.
More about...

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








