All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | PRECIS phase II trial: whole brain radiotherapy compared with intensive chemotherapy and hematopoietic stem cell rescue for young PCNSL patients
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, on December 3–6, 2016.
On Monday 5th December, an oral abstract session was held between 10:30am and 12:00pm in the “Aggressive Lymphoma (Diffuse Large B-Cell and Other Aggressive B-Cell Non-Hodgkin Lymphomas)—Results from Prospective Clinical Trials Program” category. This session was moderated by Jennifer Effie Amengual, MD, from the Columbia University Medical Center, and Lapo Alinari, MD PhD, of the Ohio State University.
Abstract #782 was titled “Whole Brain Radiotherapy (WBRT) Versus Intensive Chemotherapy with Hematopoietic Stem Cell Rescue (IC + HCR) for Primary Central Nervous System Lymphoma (PCNSL) in Young Patients: An Intergroup Anocef-Goelams Randomized Phase II Trial (PRECIS)” and was presented by Carole Soussain, MD PhD, of the Curie Institute, Hôpital René Huguenin, Saint-Cloud, France.
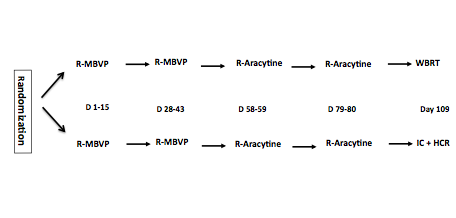
From October 2008 to February 2014, a total of 140 patients (male n=101) were recruited (median age = 55 years, range 22–60); 70 were randomized to Arm A (WBRT) or Arm B (IC+HCR) consolidation therapy. Sixty-seven patients per arm in the ITT analysis, 1 patient withdrew consent in each arm. Fifty-three patients in Arm A received WBRT and IC+HCR was administered to 44 Arm B patients. Median follow-up was 33.2 months (24.3–89.7) for Arm A and 33.8 months (23.7–64.5) for Arm B. The primary end-point was 2-year PFS (38 patients per arm).
Highlights
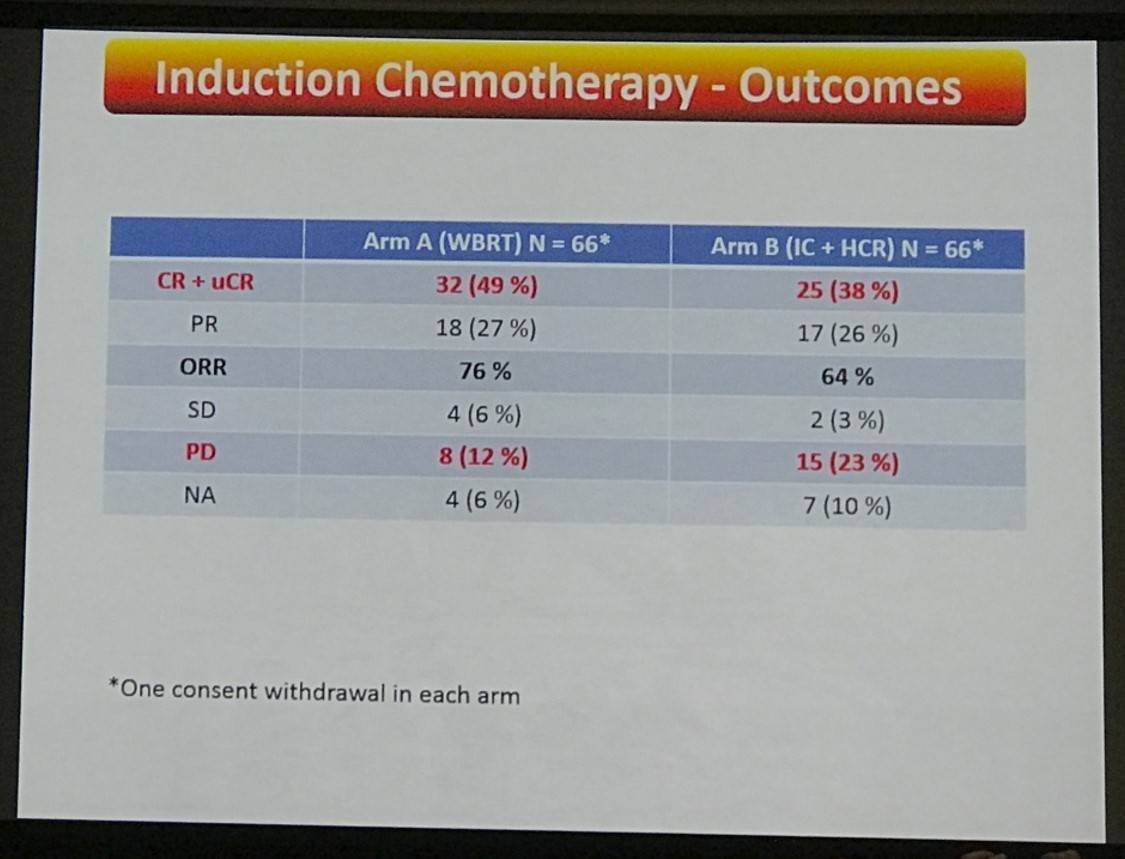
- After induction therapy with 2 cycles R-MBVP and 2 cycles R-AraC
- Arm A ORR = 76% (CR+ uCR = 49%)
- Arm B ORR = 64% (CR+ uCR = 38%)
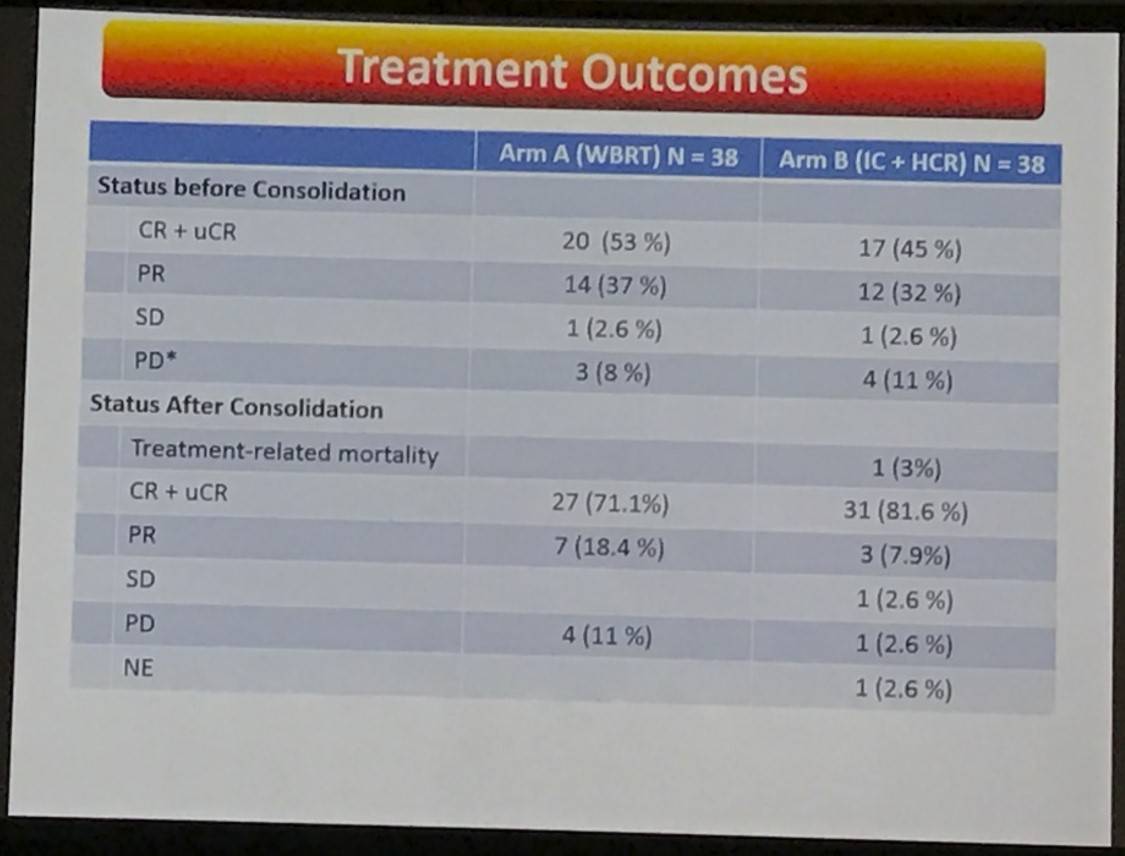
- After consolidation treatment
- Arm A = 71.1% CR+ uCR
- Arm B = 81.6% CR+ uCR
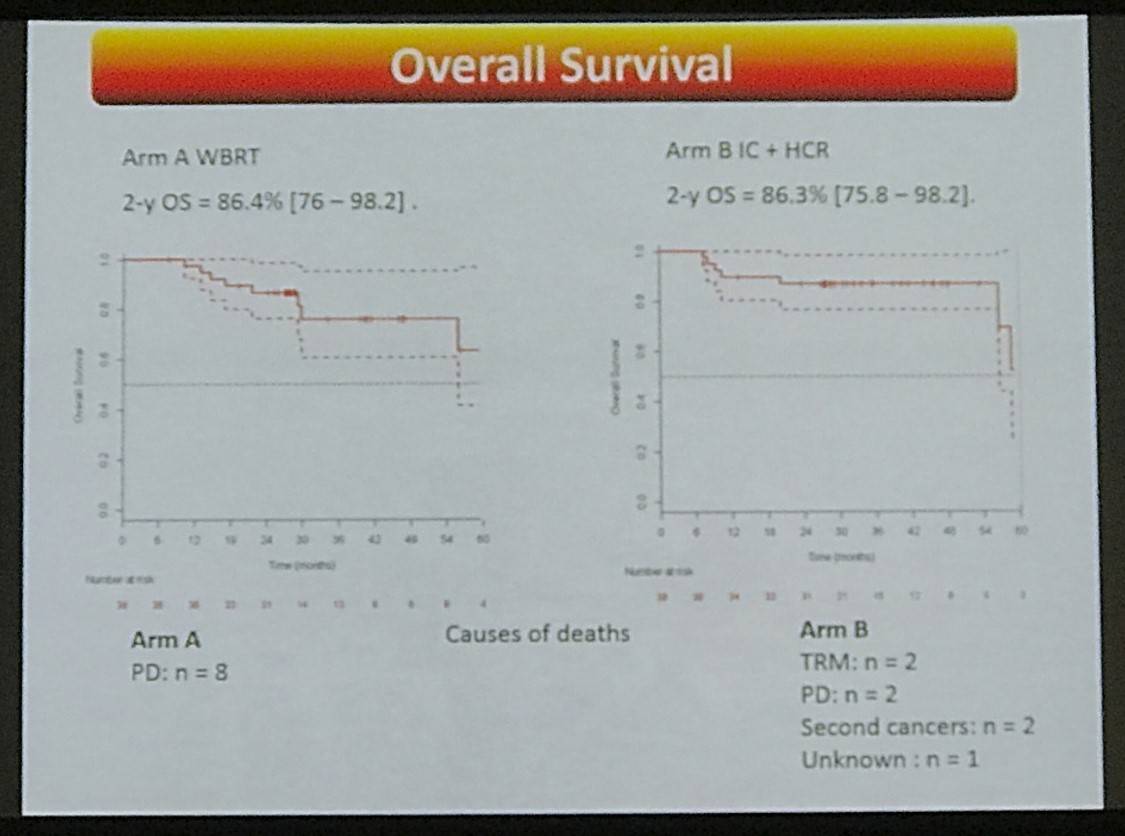
- Causes of death:
- Arm A: PD n = 8
- Arm B: TRM n = 2, PD n = 2, Secondary Malignancies n = 2, Unknown n = 1
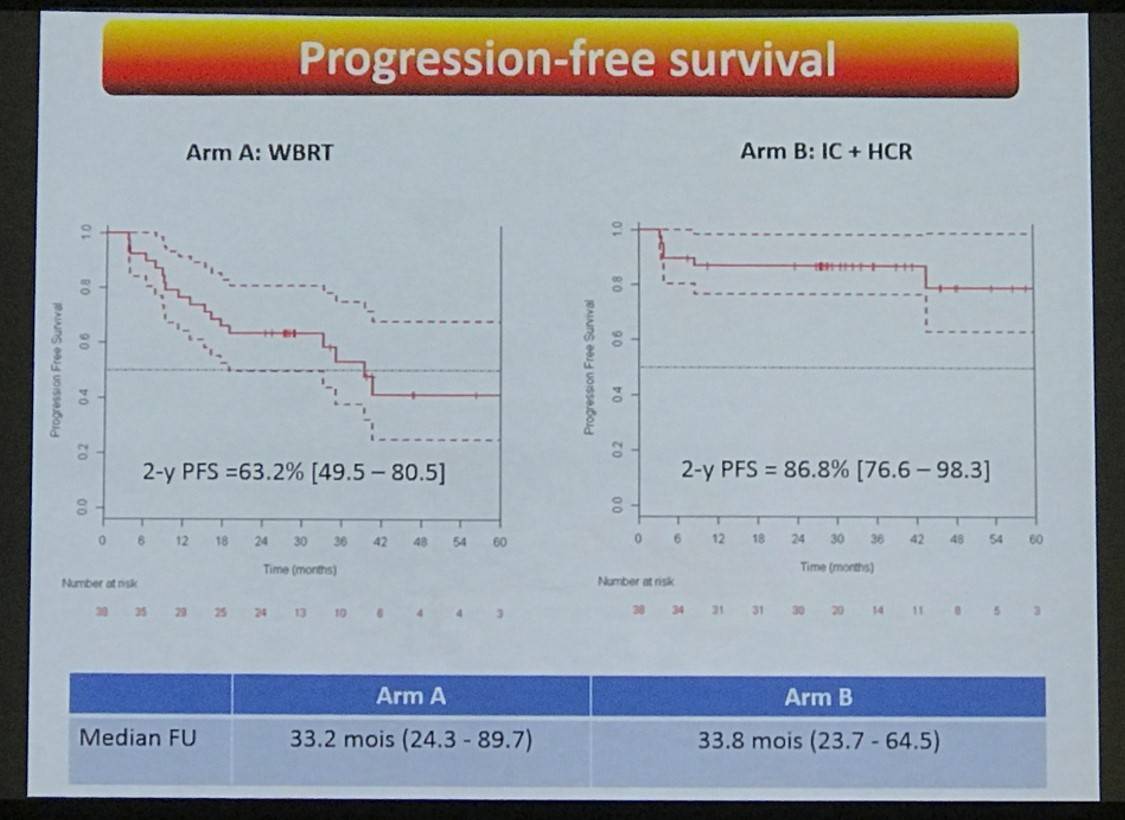
- Overall, 21 patients relapsed at the end of treatment
- Arm A: n = 16, median time to relapse = 15.1 months
- Arm B: n = 5, median time to relapse = 8.5 months
- At 2-years:
- Arm A: 14 PDs, 24 patients free of disease
- Arm B: 5 PDs + 2 TRM, 31 patients free of disease
- 2-yr PFS = 63.2% (49.5–80.5) and 86.8% (76.6–98.3) in Arms A and B, respectively
- 2-yr OS = 86.4% (76–98.2) and 86.3% (75.8–98.2) in Arms A and B, respectively
This abstract presentation concluded by stating that IC-HCR consolidation therapy showed a favorable outcome. However, ORR after induction chemotherapy still requires improvement.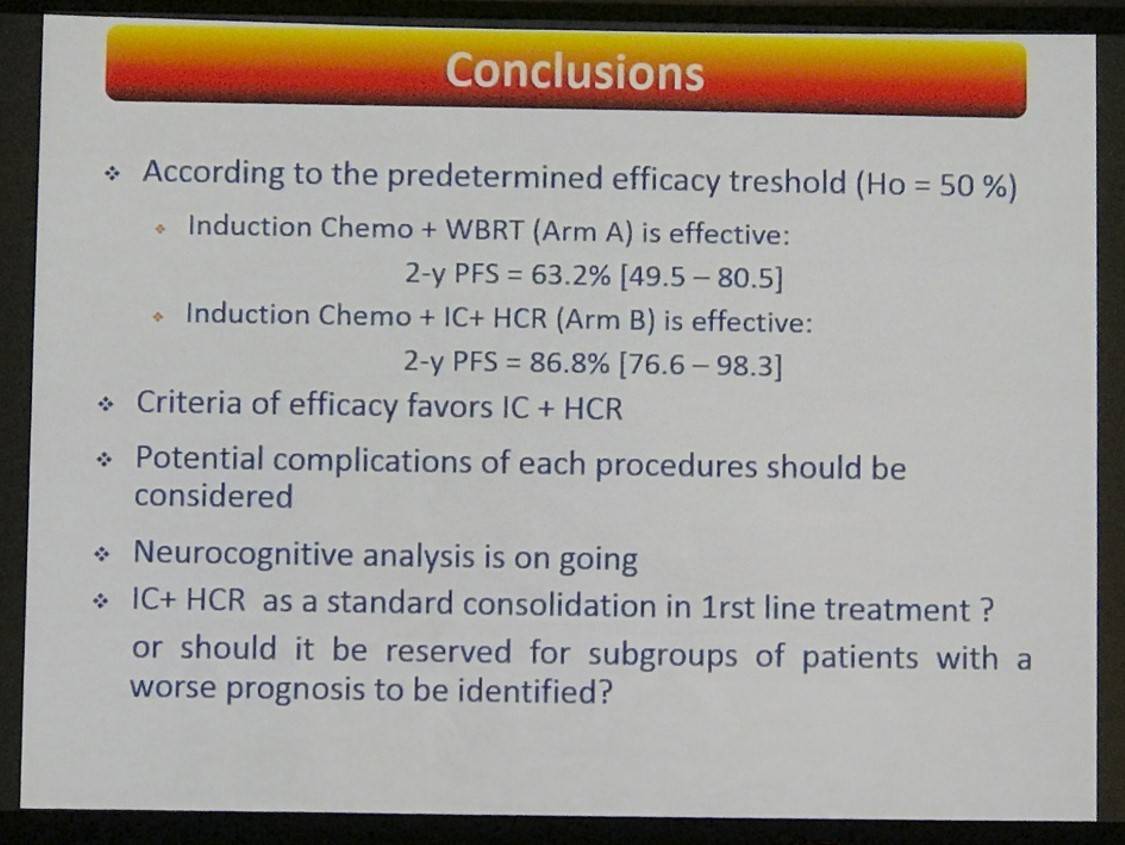
Abstract:
Background: Despite a significant improvement of therapeutic results with the current immuno-chemotherapies, a consolidation treatment is still required for decreasing the risk of relapse.
WBRT has been the historical standard consolidation in PCNSL patients, but IC + HCR has shown encouraging results in non-controlled studies. However, each procedure exposes patients to specific side effects, such as cognitive dysfunctions and treatment-related mortality, respectively. In this trial, we addressed the efficacy and toxicity of a standard chemo-immunotherapy followed by either WBRT or IC+HCR in first-line treatment of PCNSL patients.
Methods: Immuno-competent patients (aged 18-60) with newly diagnosed PCNSL and measurable disease were enrolled from 23 French centers. All the patients received 2 cycles of R-MBVP (Rituximab 375 mg/m2 D1, Methotrexate 3 g/m2 D1 and D15, VP16 100 mg/m2 D2, BCNU 100 mg/m2 D3, Prednisone 60 mg/kg/D D1-D5) followed by 2 cycles of R-AraC (Rituximab 375 mg/m2 D1, Cytarabine 3g/m2 D1-D2) as induction chemotherapy. Participants were stratified upfront according to performance status (0-1 vs 2-4) and treating institution and randomly assigned (1:1) to receive either WBRT (Arm A) or IC + HCR (Arm B) as a consolidation treatment. IC consisted in thiotepa (250 mg/mg/m2/d days -9 through -7, busulfan 10 mg/kg (total dose) days -6 through -4, and cyclophosphamide 60 mg/kg/d days -3 and -2). Prospective neuro-cognitive evaluations were planned in both arms. Responses were assessed according to the IPCG criteria. A central review of MRIs was scheduled. The primary end-point was the 2-year progression free survival (PFS). Thirty-eight assessable patients who received the complete study treatment for each treatment were needed. Either of the two arms would be deemed effective if > 24/38 patients are free of disease at 2 year with no major side effects. Analysis was intent to treat, in a non-comparative phase 2 trial design. This study is registered with ClinicalTrials.gov, number NCT00863460.
Results: Between Oct 2008 and Feb 2014, 140 patients (male: 101; female: 39) were recruited (median age = 55 years, range 22-60) and randomized in Arm A (n=70) or Arm B (n = 70). Sixty-seven patients were assessable in each arm. Two patients withdrew their consent during the induction cycles (Arm A: n = 1; Arm B: n = 1). After two cycles of R-MBVP, overall response rates were 82% (CR + uCR = 24 %) and 77 % (CR + uCR = 23 %) in arm A and arm B respectively. At the end of the induction chemotherapy, ORR was 74 % (CR+ uCR = 44 %) and 67 % (CR+ uCR = 42 %) in arm A and B respectively. WBRT was given to 53 patients in arm A and IC+HCR was given to 44 patients in arm B. After consolidation treatment, ORR was 71 % (56 % CR+ Cru) and 67 % (60 % CR+ Cru) in arm A and B respectively. Five treatment-related deaths were reported, during induction chemotherapy in arm A (n = 2) and after IC+ HCT (n = 3). Median follow-up was 27.2 and 28.6 months in arm A and arm B respectively. Relapses occurred in 21 patients after the end of treatment (arm A: n = 16; Arm B: n = 5) with a median time to relapse of 15.1 and 8.5 months in arm A and B respectively. 2-y PFS in the experimental arm was 86.8% (95 CI, 76.6 to 98.3%). 2-y PFS in arm A will be given after the completion of the ongoing MRI review in this arm. The analysis of the prospective neuropsychological evaluations is pending.
Conclusion: This study shows a favorable outcome of patients who received the IC+HCR arm. Improvement of the ORR after induction chemotherapy is still needed. The results of the 2-y PFS in arm A and of the neuropsychological evaluations are awaited to define the further standard of treatment in young patients with PCNSL.
Fundings: French Government, Roche, Pierre Fabre
- Soussain C. et al. Whole Brain Radiotherapy (WBRT) Versus Intensive Chemotherapy with Hematopoietic Stem Cell Rescue (IC + HCR) for Primary Central Nervous System Lymphoma (PCNSL) in Young Patients: An Intergroup Anocef-Goelams Randomized Phase II Trial (PRECIS). 2016 December 5; Oral Abstract #782: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








