All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Phase I/II trial finds single-agent ibrutinib is well tolerated in recurrent/refractory Primary and Secondary Central Nervous System Lymphomas
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, on December 3–6, 2016.
On Monday 5th December, an oral abstract session was held between 10:30am and 12:00pm in the “Aggressive Lymphoma (Diffuse Large B-Cell and Other Aggressive B-Cell Non-Hodgkin Lymphomas)—Results from Prospective Clinical Trials Program” category. This session was moderated by Jennifer Effie Amengual, MD, from the Columbia University Medical Center, and Lapo Alinari, MD PhD, of the Ohio State University.
Abstract #783 was titled “Single-Agent Ibrutinib in Recurrent/Refractory Central Nervous System Lymphoma” and was presented by Christian Grommes, MD, of the Memorial Sloan Kettering Cancer Center, New York, NY.
This phase 1/2, open-label, single-institution study included patients aged 18 years or over with R/R Primary CNS Lymphoma (PCNSL) or Secondary CNS Lymphoma (SCNSL). Patients who had received prior allogeneic stem cell transplantation were excluded. In total, 20 patients were enrolled (PCNSL n=13; SCNSL n=7) with a median age of 69 years (21–85) and a median of 2 prior therapies (1–8). MTX-based salvage therapy had failed in 75% of patients.
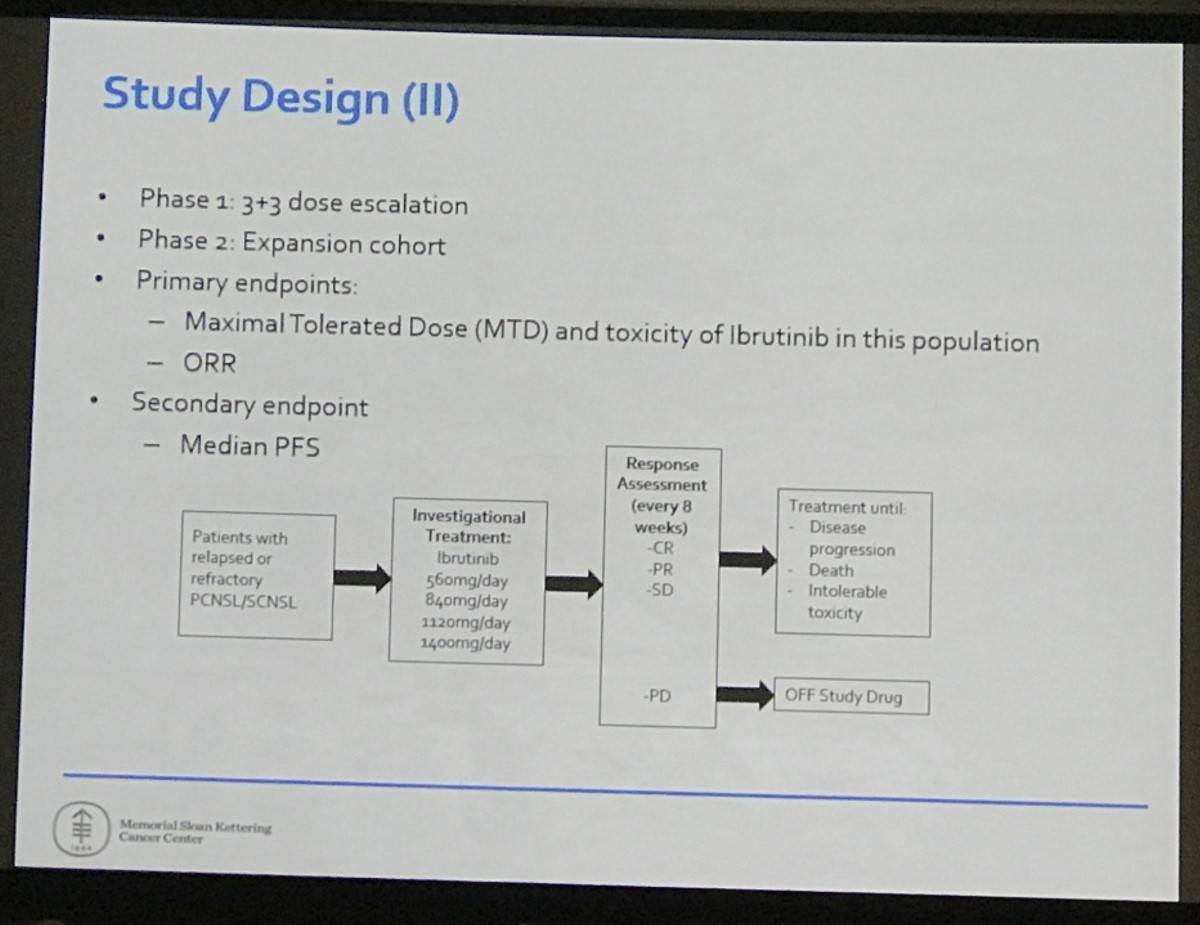
- Adverse events:
- No dose limiting toxicities were observed
- Six patients experienced 3 grade 4 AEs: lymphopenia, sepsis and neutropenia
- Nine patients experienced grade 3 AEs
- The most frequently reported AEs: hyperglycemia, hypertriglyceridemia, thrombocytopenia, and anemia
- One patient stopped treatment (aspergillosis of lung and brain)
- One patient had dose reduced (colitis)
- Pharmacokinetics in the Cerebrospinal Fluid (CSF)
- Mean ibrutinib CSF concentrations = 0.77ng/mL (1.7nM) and 1.96ng/ML (4.4nM) in the 560mg and 840mg arms, respectively
- Drug concentrations increased at steady state
- Meaningful CSF ibrutinib concentrations were reached
- At median follow-up of 255 days (147–532)
- 19/20 patients were evaluated for response
- A response was reported in 15/20 (75%) patients: 10 CR (3 in CSF only), 5 PR; PCNSL: 10/13 (76%); SCNSL 5/7 (71%)
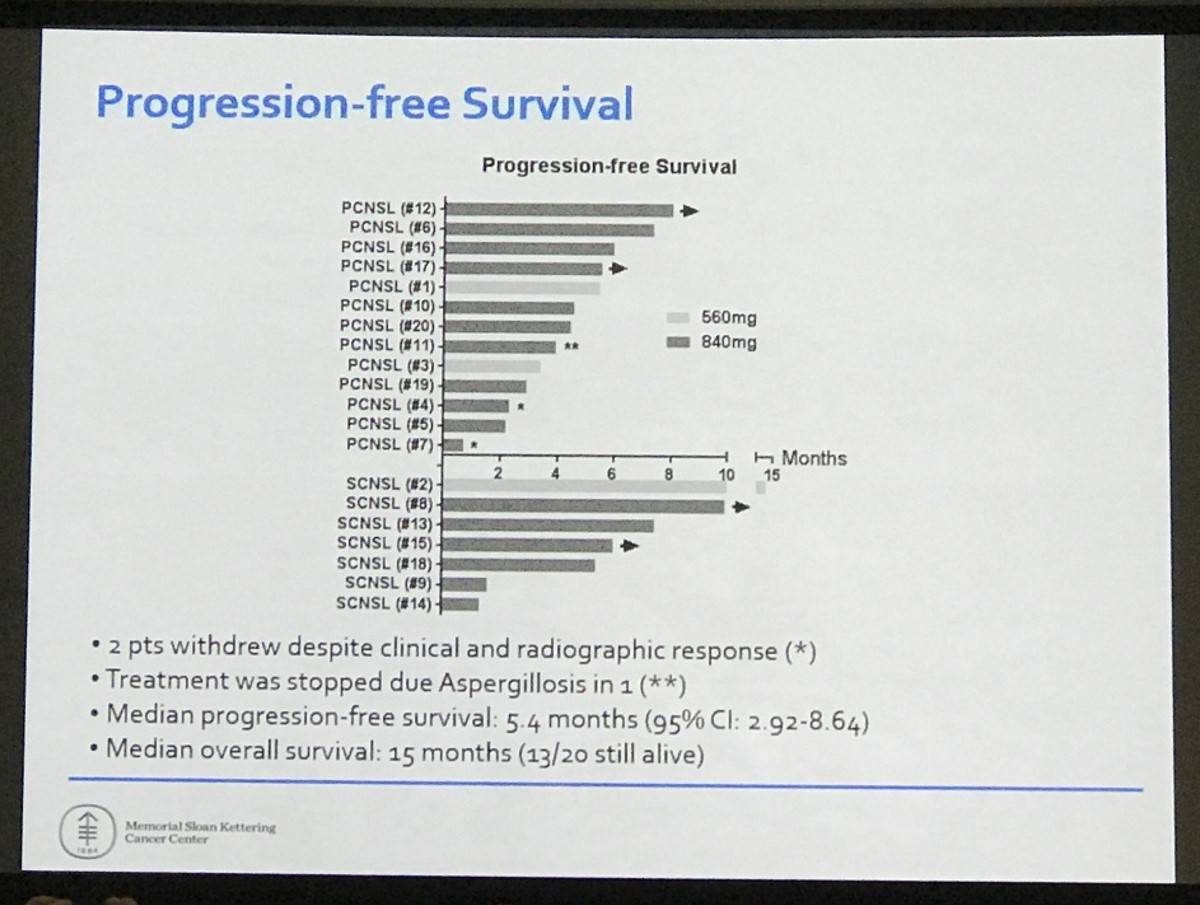
- Median PFS = 5.4 months (95% CI: 2.92–8.64)
- Median OS = 15 months (13/20 still alive)
- 10/20 (50%) required corticosteroids at enrollment; 6/10 (60%) were successfully tapered off dexamethasone after initiating ibrutinib
This abstract presentation concluded by stating that ibrutinib treatment is well tolerated in patients with Primary and Secondary CNS Lymphomas who experienced manageable AEs. A combination trial of ibrutinib plus methotrexate with or without rituximab is ongoing.

Abstract
BACKGROUND: Primary Central Nervous System Lymphoma (PCNSL) is an aggressive primary brain tumor with median progression free survival (PFS) after upfront methotrexate-based chemotherapy of 2-3 years. Outcome and treatment options are poor for recurrent/refractory (r/r) disease. Response rates (ORR) range between 30-60% with a PFS of 2-5 months. Ibrutinib has shown promising clinical response in Mantel cell lymphoma, CLL, and Waldenström. This trial investigates Ibrutinib in patients with r/r PCNSL and SCNSL.
METHODS: Eligible patients had r/r PCNSL or Secondary CNS Lymphoma (SCNSL), age≥18, ECOG≤2, normal end-organ function, and unrestricted number of CNS directed prior therapies. In patients with SCNSL disease, systemic disease needed to be absent.
RESULTS: Twenty patients were enrolled (3 at 560 mg; 17 at 840 mg). Median age was 69 (range 21-85); 12 were women. Median ECOG was 1 (0: 2, 1: 12, 2: 6). 65% had PCNSL and 35% SCNSL; 70% had recurrent disease. Eleven had parenchymal disease, 3 isolated cerebrospinal fluid (CSF) involvement and 6 both. Five grade 4 adverse events were observed in 4 patients (lymphopenia (2), sepsis (1), neutropenia (2)). Ten patients developed grade 3 toxicities, including lymphopenia in 3 patients, thrombocytopenia in 2, hyperglycemia in 2, lung infection in 2, neutropenia in 1, urinary tract infection in 1, colitis in 1, and fungal encephalitis in 1. The most common toxicities were hyperglycemia, anemia, and thrombocytopenia. After a median follow-up of 193 days, 19/20 patients were evaluated for response: 8 CR, 7 PR, 1 SD and 3 PD; 75% (15/20) ORR. The median PFS is 7.29 months (95% CI: 3.80-15.43 months (longest: 15.3 months)). The mean Ibrutinib concentration in the CSF 2h post administration at day 1 and 29 is 1.75 ng/mL (3.97 nM) and 2.51 ng/mL (5.6 nM) which is above the IC50 (1nM) required in vitro to reduce growth of lymphoma cells. An additional treatment arm has been added to the trial which will evaluate adverse events of the combination of ibrutinib and high-dose methotrexate chemotherapy. Enrollment into the combination arm is ongoing and updates will be presented at the meeting.
CONCLUSION: Patients with CNS lymphoma tolerate Ibrutinib with manageable adverse events. Drug concentrations in CSF are higher at steady state (day 29) and meaningful CSF concentrations are reached. Clinical response was seen in 75% of CNS lymphoma patients. A combination arm will assess the adverse events of ibrutinib in combination with high-dose methotrexate chemotherapy.
- Grommes C. et al. Single-Agent Ibrutinib in Recurrent/Refractory Central Nervous System Lymphoma. 2016 December 5; Oral Abstract #783: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








