All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ASH 2016 | Rituximab-Lenalidomide (REVRI) in Relapse or Refractory Primary Central Nervous System or Vitreo-Retinal Lymphoma: Results of a “Proof of Concept” Phase II Study of the French LOC Network
Bookmark this article
The 58th Annual Meeting & Exposition of the American Society of Hematology’s (ASH) took place in San Diego, CA, and on December 5th, Carole Soussain, MD, PhD, from Department of Hematology, Curie Institute, Hôpital René Huguenin, Saint-Cloud, France, presented interim data from a prospective, multi-center, open-label phase-II study conducted by the French LOC Network into the activity of rituximab-lenalidomide in treatment of R/R PCNSL or Primary Vitreo-Retinal Lymphoma (PVRL).
Highlights:
- 50 pts with R/R PCNSL (n=42pts) or PVRL (n=8pts)
- Treatment: eight 28 day cycles of R2 (375mg/m2 IV rituximab on day 1, + lenalidomide 20mg per day for days 1-21, increased to 25mg after first cycle) followed by twelve 28 day cycles maintenance lenalidomide at 10mg per day on days 1-21
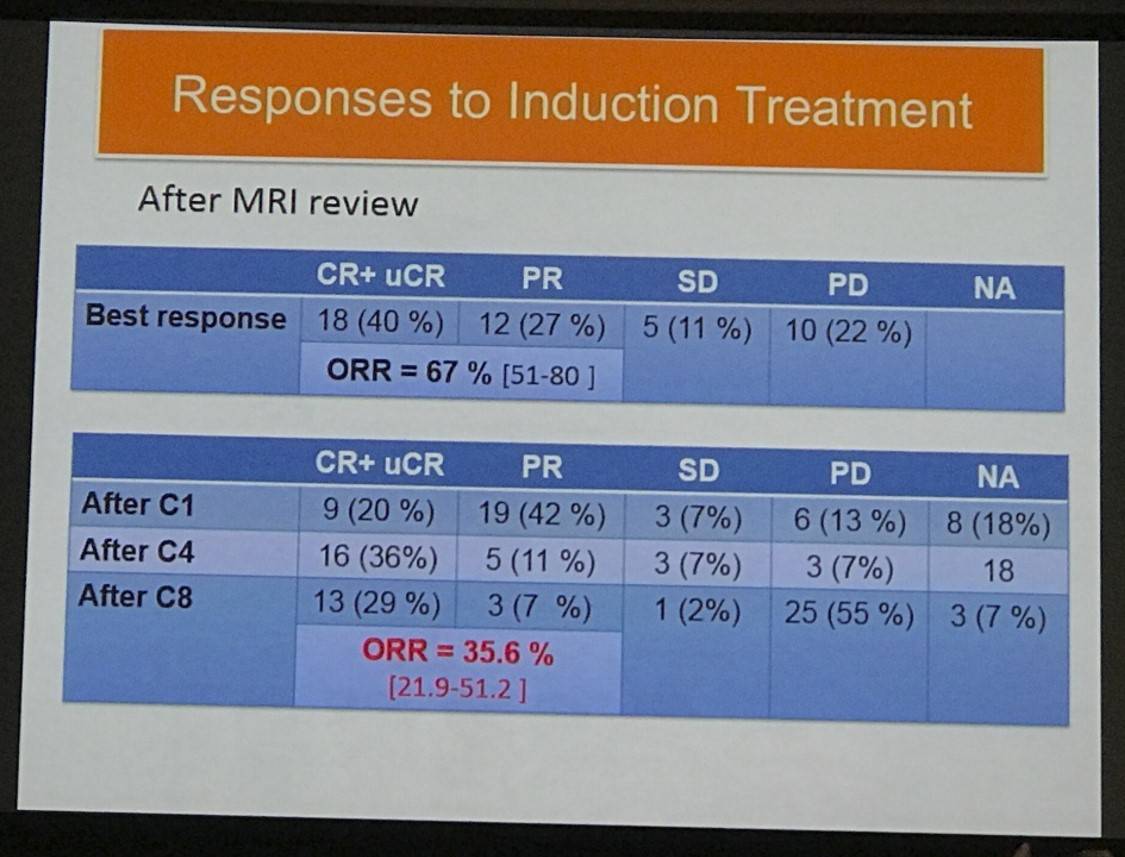
- Responses to Induction Treatment after MRI review
- ORR = 35.6% (21.9–51.2)
- Best response ORR = 67% (51–80)
- CR + uCR = 13 (29%)
- Best response CR + uCR = 18 (40%)
- Maintenance phase = 17pts
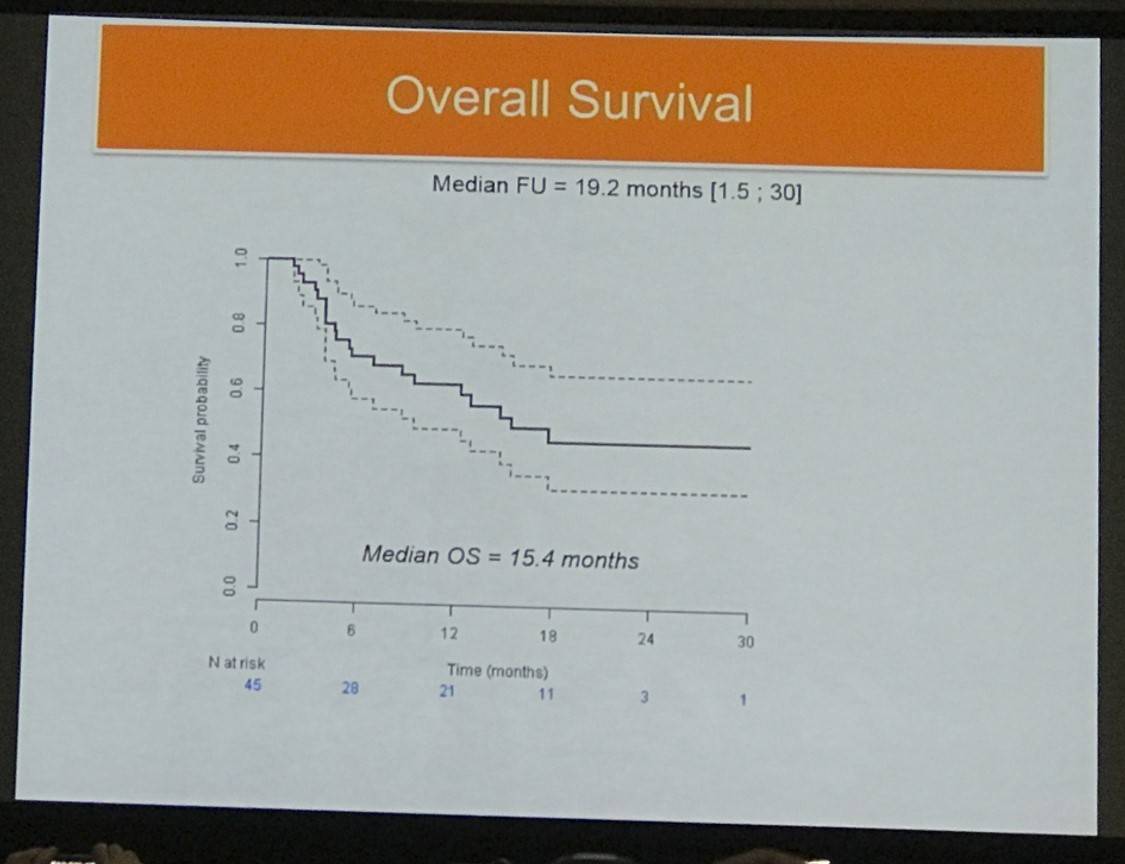
- Median OS =15.4 months (median follow-up = 19.2 months [1.5–30])
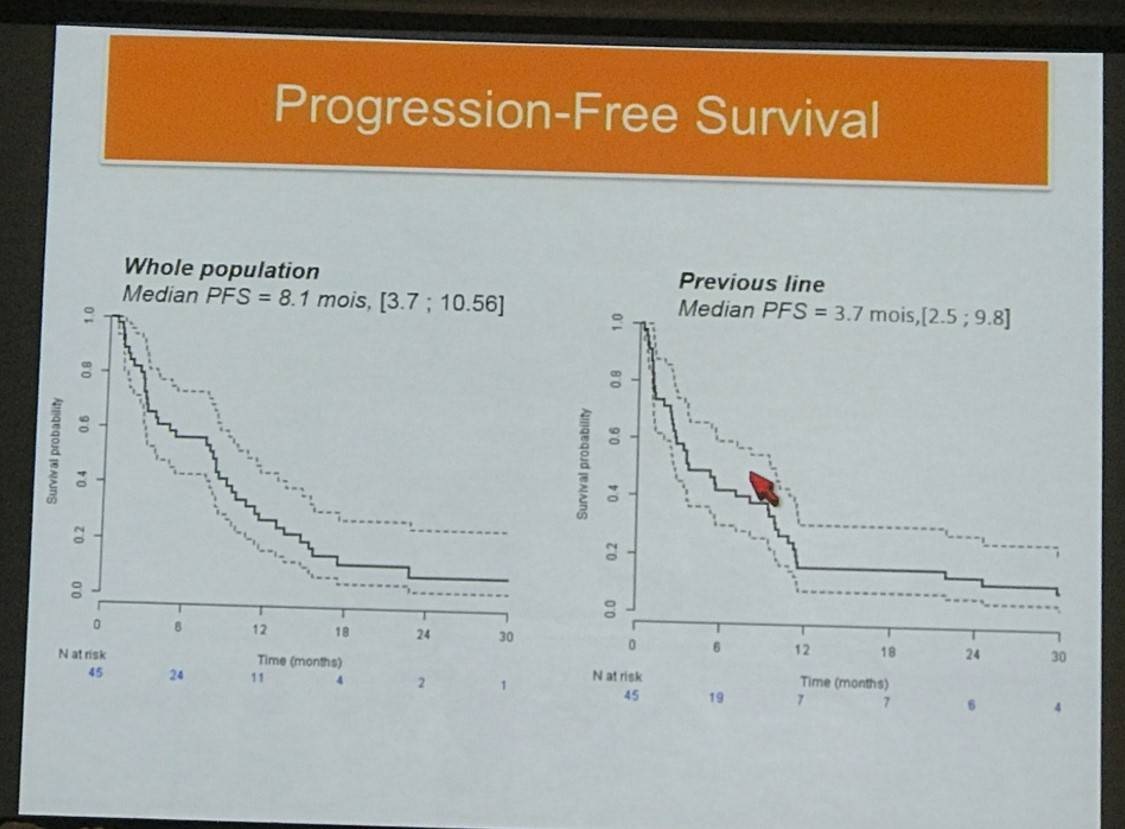
- Median PFS of whole population= 1 months (3.7–10.56)
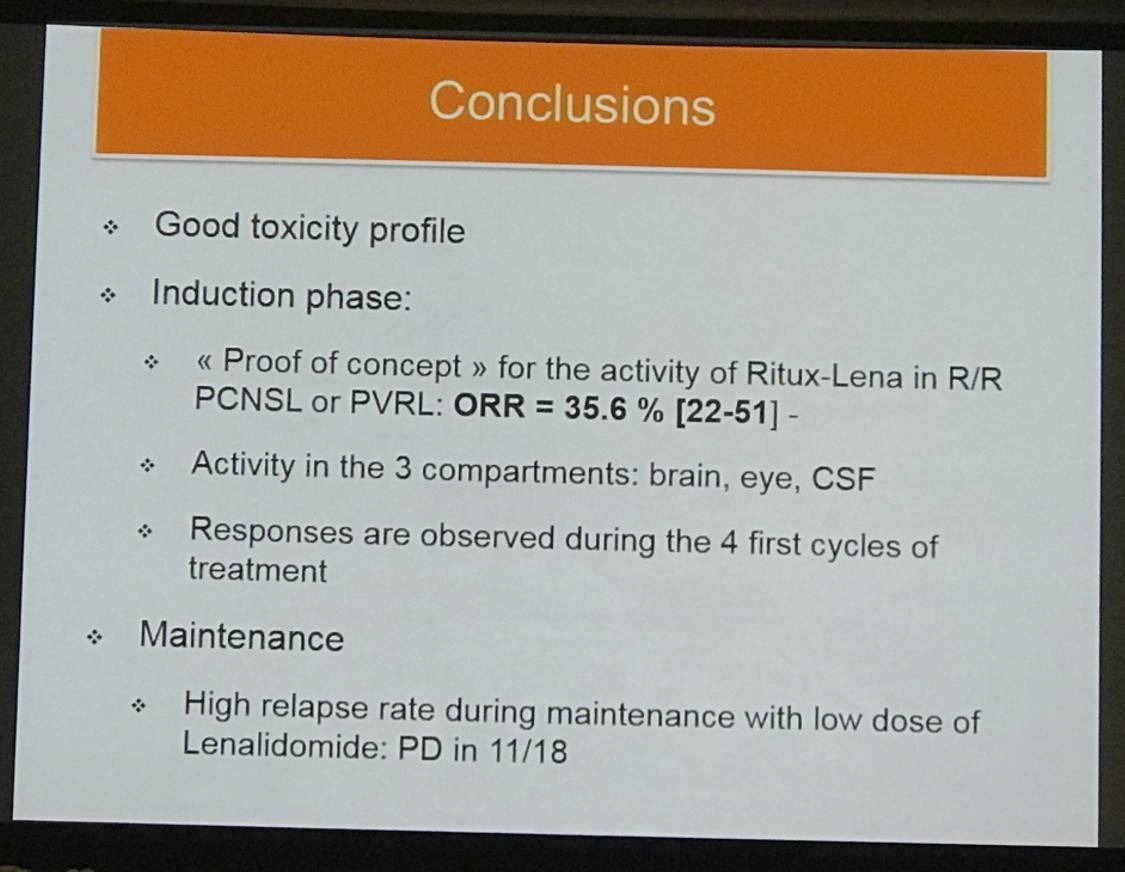
In conclusion, rituximab-lenalidomide has been shown to be active in R/R PCNSL or PVRL patients, however updated results with a longer follow up time are required before firm conclusion can be reached, however further exploration of this treatment option is desirable.
Abstract:
Background. Primary CNS lymphoma (PCNSL) is a diffuse large B-cell lymphoma (DLBCL), predominantly of non-germinal center (non-GC) subtype. Despite improvement of therapeutic results since the introduction of high-dose methotrexate (MTX) in first-line treatment, improvements of therapeutic results are needed in PCNSL, both in first-line treatment and at relapse. The roles of consolidation and maintenance therapy need to be addressed as well. The association of Rituximab and Lenalidomide has shown activity in non-CNS non-GC DLBCL, but its activity in PCNSL was unknown.
Methods. In this prospective, multicenter open-label phase II study, we enrolled patients over 18 with a refractory or relapse PCNSL or primary vitreo-retinal lymphoma (PVRL) of DLBCL type. The treatment consisted in an induction phase with 8 cycles of 28 days with R2 (Rituximab 375/m2 IV D1; Lenalidomide 20 mg/D D1-D21 for the first cycle then 25 mg/D D1-D21 for the subsequent cycle in the absence of hematologic toxicity), followed, in responder patients by a maintenance phase with 12 cycles of 28 days with Lenalidomide alone (10 mg/D, D1-D21). Corticosteroids were allowed during the first induction cycle in case of a threatening or symptomatic edema. Deep vein thrombosis prophylaxis was mandatory. Patient who received a complete month of treatment were evaluable for response. The primary end-point was the objective response rate (ORR) at the end of the induction phase, according to the international primary CNS lymphoma collaborative group (IPCG) criteria. The analysis was based of a Fleming's two-step design (P0 = 10 %; P1= 30%). A pilot exploration of circulating NK andT cells before and after treatment and correlation with therapeutic response was conducted. This study is registered with ClinicalTrials.gov, number NCT01956695.
Results. Between September 17, 2013 and September 29, 2015, fifty patients (median age: 69, range 46-86) were recruited from 10 centers. All the patients had previously received high-dose (HD) MTX. Median number of previous lines of chemotherapy was 2 (range, 1-4). Nine patients had previous received an HD chemotherapy followed by hematopoetic stem cell rescue. Initial diagnoses were PCNSL (n = 42) and PVRL (n = 8). At time of inclusion in the study, diagnoses were PCNSL (n = 41) and PVRL or isolated intra-ocular (IO) relapse of PCNSL (n=9). Seven patients had concomitant involvement of the cerebrospinal fluid (CSF). Patients were included either for a relapse after last treatment (80 %; median time to relapse = 5.5 months), or for a refractory disease (20 %). Thirty-four patients received concomitant corticosteroids during the first month of treatment. Forty-five patients were evaluable for response after the first cycle of treatment. Median number of induction cycles was 7 (range, 1-8). Grade 3 or 4 adverse events were reported in 11 patients (infection: n = 10, cutaneous rash: n =1). A second cancer (melanoma) occurred in one patient. Two patients withdrew their consent. During the induction phase, best observed responses were CR (n = 16), PR (n = 11), stable disease (n = 5) and progressive disease (n = 11) for an ORR of 63% (27/43). . At the end of the induction phase, 43 patients were evaluable for the primary objective. ORR was 39 % (17/43) including 13 CR (30%). A response has been observed in patients included for a PCNSL (n = 13, 32%) and for an IO relapse or PVRL (n = 4, 44%). Seventeen patients started the maintenance phase. With a median follow-up of 9 months (range, 1.1-15.4), median overall and progression-free survivals of the whole population were 15.3 months (95 % CI, 9.6 – non reached) and 8.1 months (95 % CI, 4.2 - non reached) respectively. Median duration of response in the responder patients was 8.9 months (95 % CI, 7.6- non reached). The results of the maintenance phase are pending. Results will be further updated.
Conclusion. This phase II study demonstrates a significant activity of the rituximab-lenalidomide regimen in relapse or refractory PCNSL or PVRL. Updated results with a longer follow-up are awaited to better evaluate the PFS and the median duration of response. This regimen warrants to be added in the armamentarium drugs for PCNSL and further explored in combination with other chemotherapies in first-line treatment, as maintenance therapy, or as a chemo-free regimen for patients unfit for high-dose methotrexate.
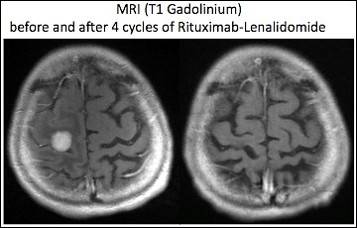
- Soussain C. et al. Rituximab-Lenalidomide (REVRI) in Relapse or Refractory Primary Central Nervous System (PCNSL) or Vitreo Retinal Lymphoma (PVRL): Results of a “Proof of Concept” Phase II Study of the French LOC Network. 2016 December 5; Oral Abstract #785: ASH 58th Annual Meeting and Exposition, San Diego, CA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








