All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
iwCLL 2017 | Baseline left atrial abnormality independently predicts atrial fibrillation in CLL patients treated with ibrutinib
Bookmark this article
On 14th May 2017, during iwCLL, the fifth session took place titled “Additional Considerations for the Initial Treatment of CLL.” This session was chaired by Richard Furman (Weill Cornell) and Jae Park (Memorial Sloan Kettering Cancer Center).
During this session, a talk titled “Left Atrial Abnormality as a Predictor of Ibrutinib-Associated Atrial Fibrillation in Patients With Chronic Lymphocytic Leukemia: a Predictive Model” was given by Anthony Mato from the Hospital of the University of Pennsylvania, Philadelphia, PA, USA.
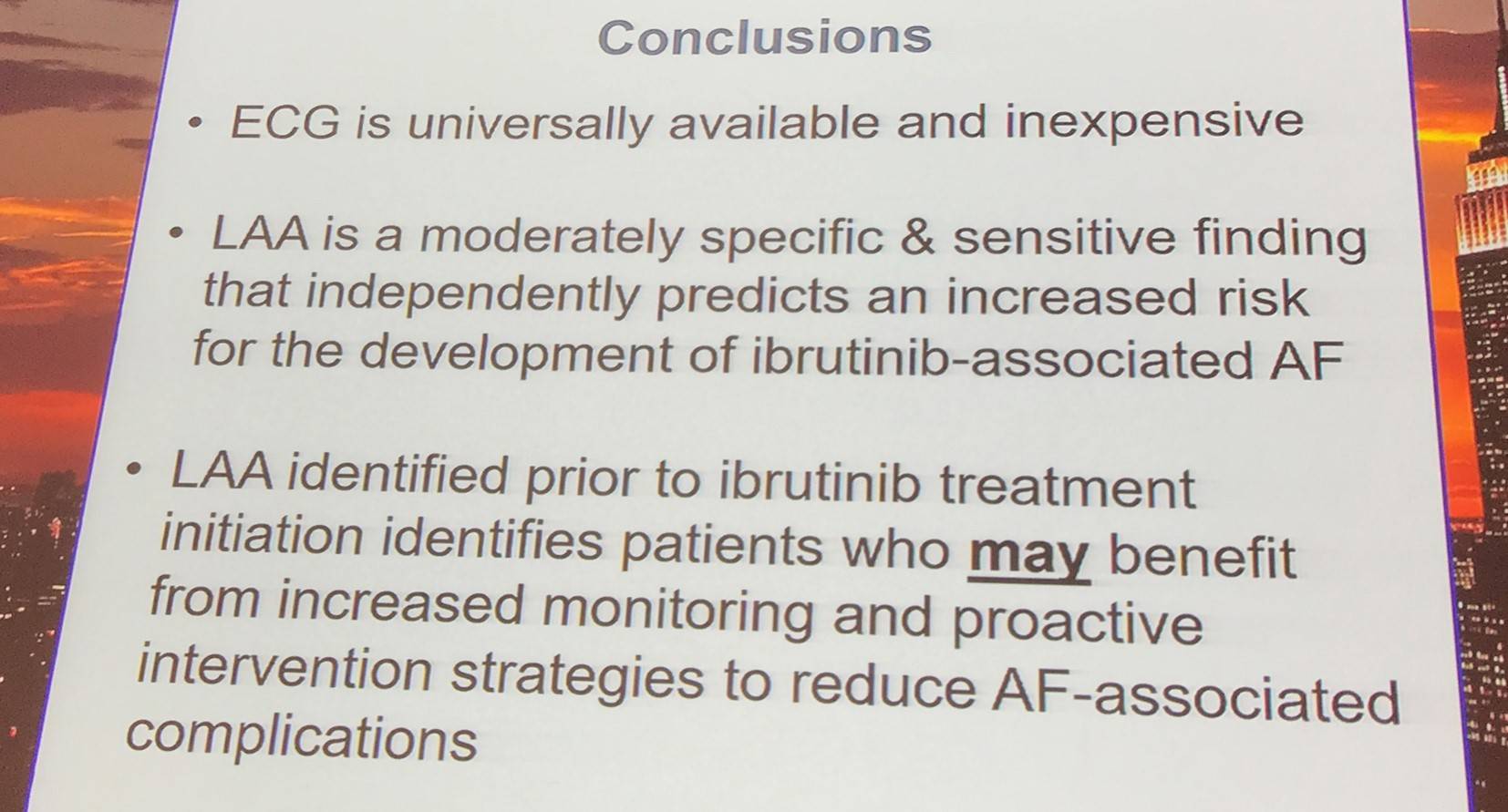
Results from long-term follow-up data indicate an approximate incidence of Atrial Fibrillation (AF) of 9–11% in CLL patients treated with ibrutinib. AF is the most frequent cause of ibrutinib interruption or discontinuation. Patients with AF are also more likely to experience Congestive Heart Failure (CHF), embolic Cerebrovascular Accident (CVA), and bleeding events. A higher risk of AF is associated with Hypertension (HTN), older age, mitral valve disease, and CHF. Left Atrial Abnormality (LAA) detected by ECG indicates remodeling of the left atrium (fibrosis, microscopic myocyte hypertrophy), which has been associated with AF development.
Anthony Mato presented results from a case-controlled, retrospective study of patients with CLL treated at two centers. Patients with pre-ibrutinib AF, no pre-treatment ECG, and receiving less than 420mg ibrutinib daily were excluded. The primary outcome was AF during ibrutinib therapy and secondary outcomes included time from ibrutinib exposure to AF, incidence of ibrutinib-associated HTN, and the association between baseline cardiovascular characteristics and AF.
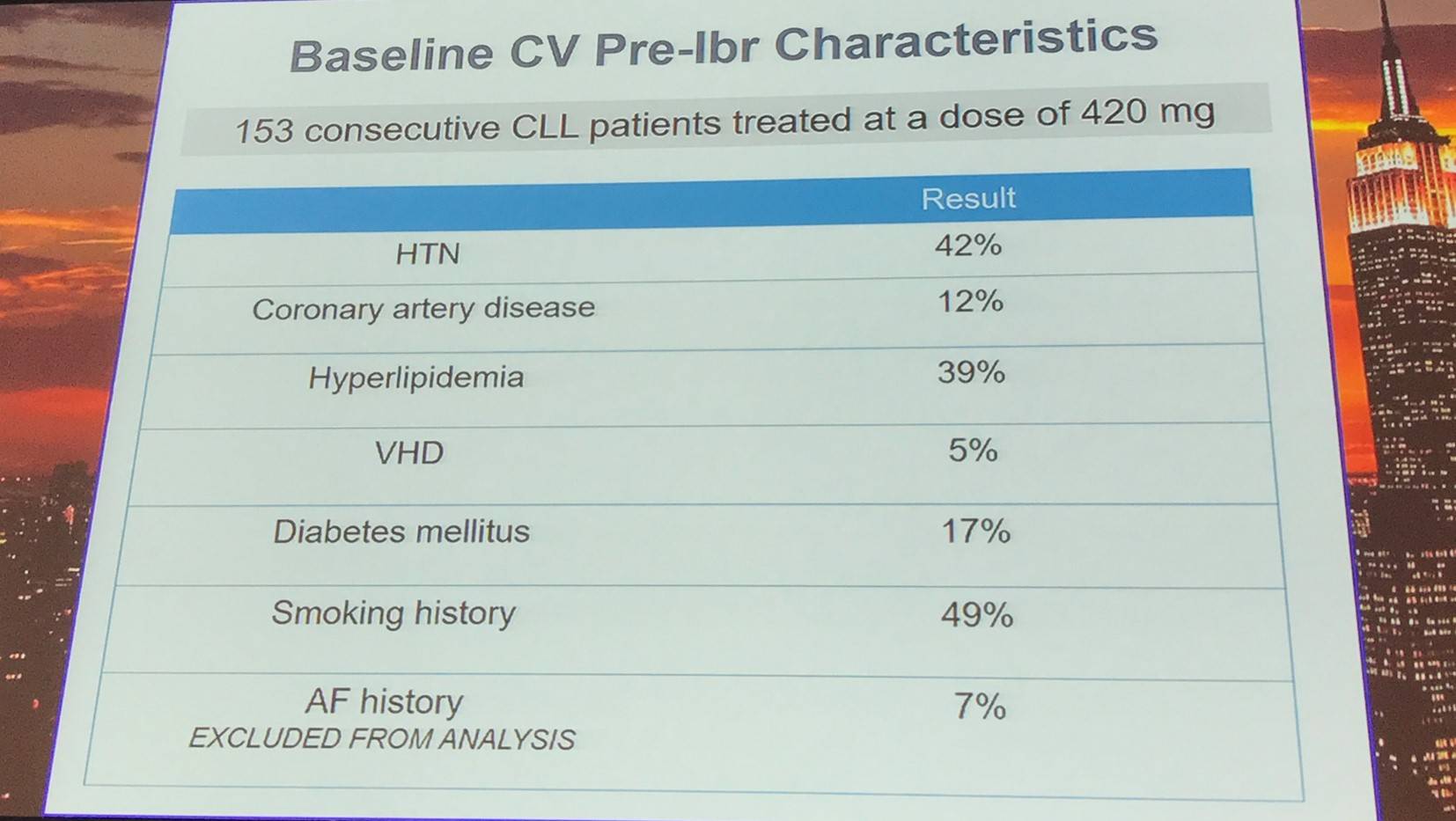
Overall, 153 consecutive patients with CLL were treated at a dose of 420mg ibrutinib:
|
Characteristic |
Result (range) |
|---|---|
|
Median age at diagnosis |
61 |
|
Median age at ibrutinib initiation |
70 |
|
Media prior therapies |
2 (0–10) |
|
R/R CLL |
87% |
|
Del(17p) |
28% |
|
Del(11q) |
32% |
|
Complex karyotype (≥3) |
35% |
|
Median follow-up |
17 months |
|
Median time from diagnosis to ibrutinib |
73 months |
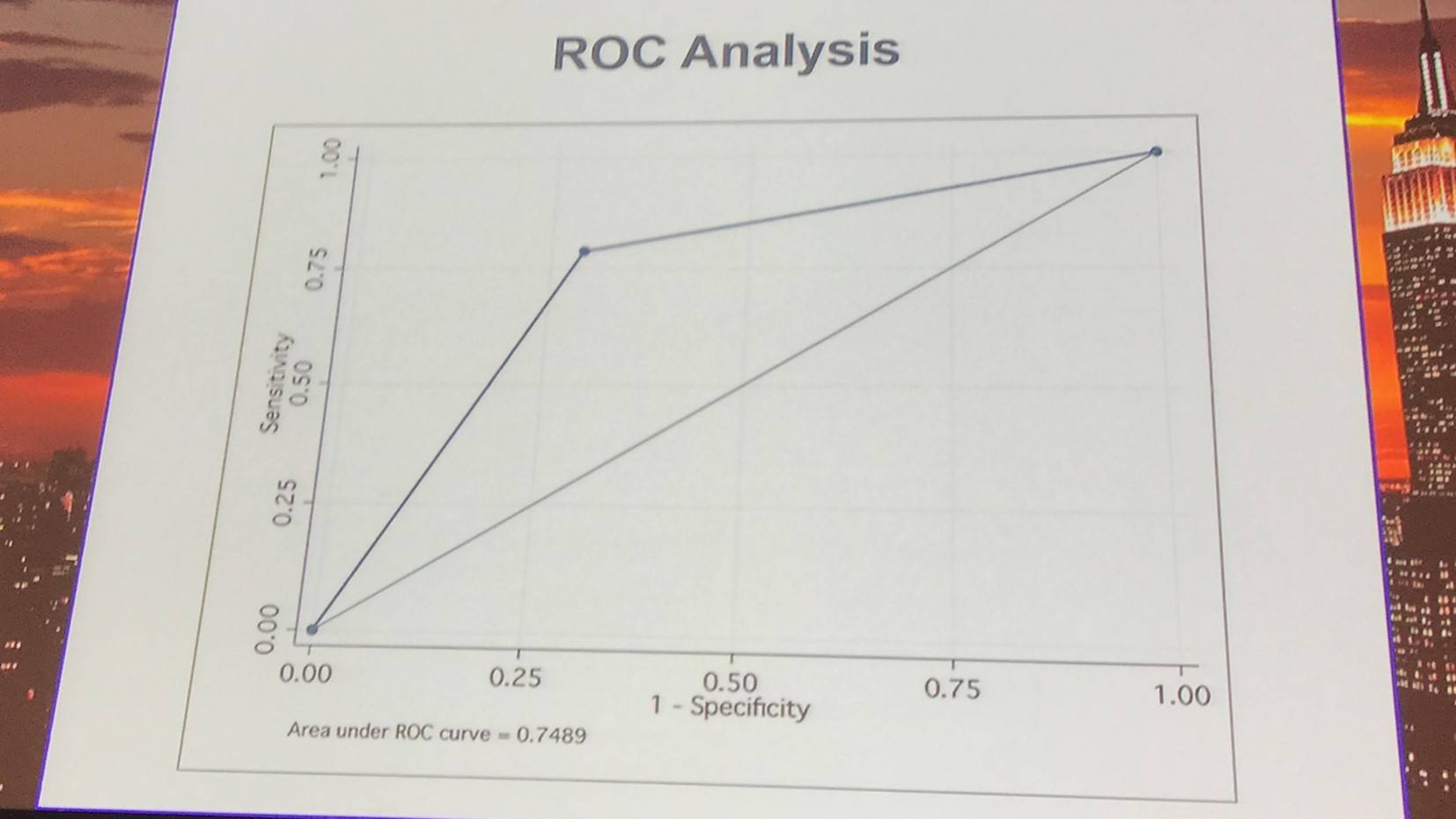
In total, 44 patients with pre-ibrutinib ECG were selected for LAA analysis: 20 cases and 24 controls. On univariate and multivariate analysis, baseline LAA independently predicted ibrutinib-associated AF:
- Univariate: OR = 9.1 (95% CI, 2.2–37.3; P = 0.002)
- Multivariate: OR = 6.6 (95% CI, 1.5–29.2; P = 0.01)
The test characteristics of the association between LAA and AF were also presented:
|
Characteristic |
Value |
95% CI |
|---|---|---|
|
Sensitivity |
79% |
54–94% |
|
Specificity |
71% |
49–87% |
|
Positive likelihood ratio |
2.7 |
1.4–5.3 |
|
Negative likelihood ratio |
0.30 |
0.1–0.74 |
|
Positive predictive value |
68% |
45–86% |
|
Negative predictive value |
81% |
58–95% |
No patient required electrophysiological intervention including cardioversion, ablation, or left atrial appendage occlusion device. The methods of AF management carried out during the study were presented:
|
Pharmacologic intervention |
Percentage |
|---|---|
|
ASA or anti-platelet |
40% |
|
Systemic anti-coagulation |
45% |
|
Anti-arrhythmic agent |
45% |
|
Rate control |
90% |
|
ACE/ARB |
40% |
|
Diuretic |
25% |
Anthony Mato then discussed limitations of the study which included the retrospective nature of the study, the fact that the cases and controls were not balanced for baseline cardiovascular characteristics, the small number of AF cases included, and the variable timing of pre-ibrutinib ECG. In addition, the results require validation in prospective studies.

- Mato A. Left Atrial Abnormality as a Predictor of Ibrutinib-Associated Atrial Fibrillation in Patients With Chronic Lymphocytic Leukemia: a Predictive Model. XVII International Workshop on Chronic Lymphocytic Leukemia; 2017 May 12–15; New York, USA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








