All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
An expert panel hosted by

Sequencing immune-based therapies in B-cell malignancies
with Ulric Jäger, Sagar Lonial, and Krina Patel

Saturday, June 15 | 18:00-19:30 CEST
Register nowThis independent education activity is sponsored by Bristol Myers Squibb. All content is developed independently by the faculty. Funders are allowed no direct influence on the content of this activity.
The Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Camrelizumab plus GEMOX as salvage therapy for R/R cHL: Results from a phase II trial
Bookmark this article
For patients with relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL), standard salvage therapy consists of high-dose chemotherapy followed by autologous stem cell transplantation (auto-HSCT); however, the efficacy of conventional salvage chemotherapy treatment can vary.1 Camrelizumab, a humanized IgG4 monoclonal antibody targeting PD-1, has demonstrated efficacy in patients with R/R cHL based on long-term results from a phase II trial (NCT03155425).1
Here, we summarize results from a phase II trial (NCT04239170) assessing the safety and efficacy of camrelizumab, in combination with the gemcitabine and oxaliplatin (GEMOX) chemotherapy regimen, followed by auto-HSCT in patients with R/R cHL, recently published Liu et al.1 in BMC Medicine.1
Study design and patient population1
- This was a single-arm, open-label trial
- Patients were transplant-eligible, aged ≥18 years, and had R/R cHL
- Patients received two biweekly cycles of 200 mg camrelizumab, followed by 4-week cycles of 200 mg camrelizumab, 1,000 mg/m2 gemcitabine, and 100 mg/m2 oxaliplatin on Days 1 and 15
- Patients with a complete response (CR) or a partial response (PR) following an additional cycle could proceed to auto-HSCT
- The primary endpoint was CR rate
Key findings1
Patient characteristics
- In total, 42 patients were enrolled
- Median age was 34 years (range, 21–58 years)
Treatment response
Upon completion of the protocol therapy, the CR rate was 64.3% (Figure 1).
Figure 1. Response rates after protocol therapy*
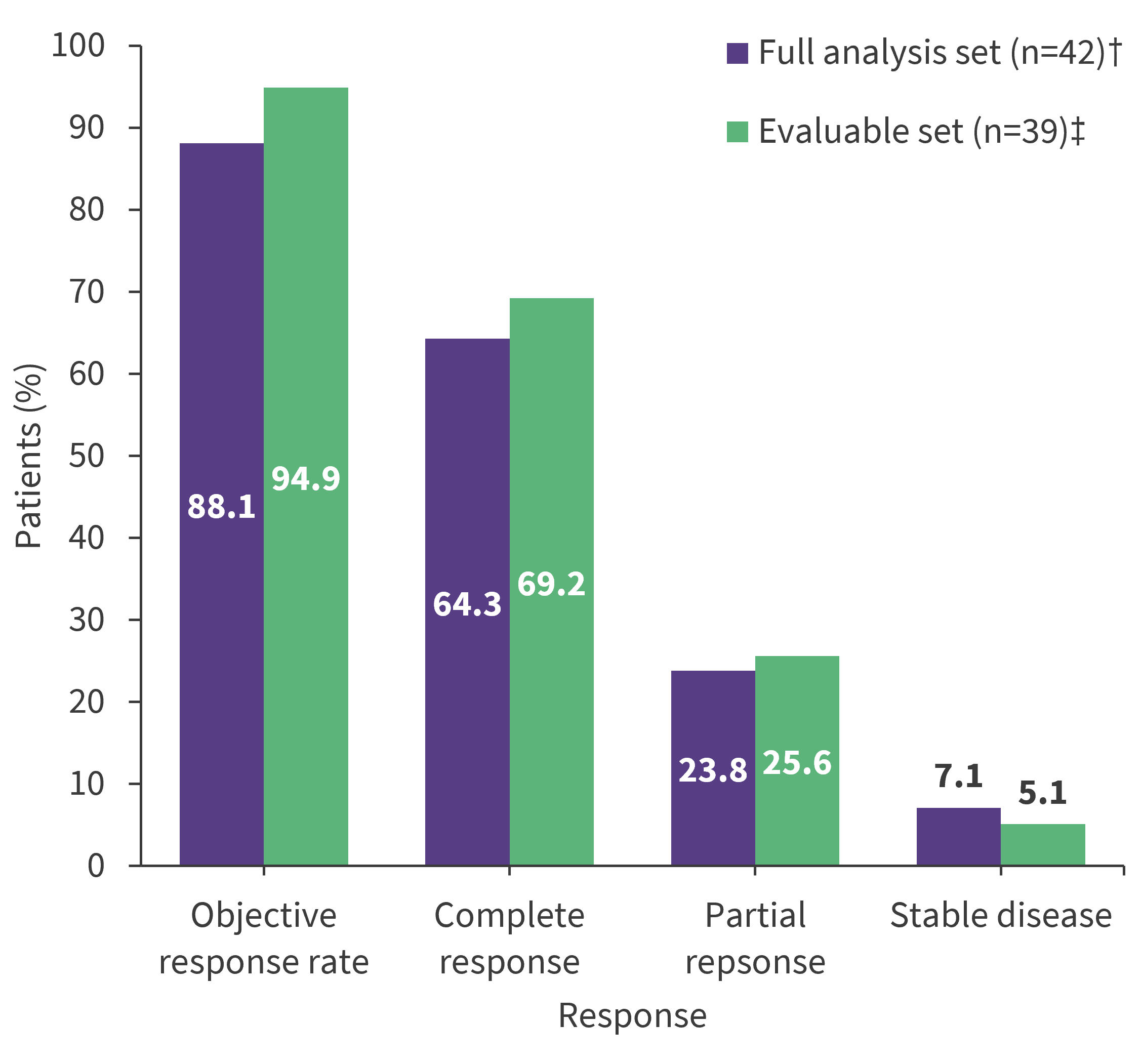
*Data from Liu, et al.1
†Includes all patients who received at least one dose of the study drug.
‡Includes patients with at least one posttreatment response evaluation.
Auto-HSCT
- In total, 29 patients (CR, n = 24; PR, n = 5) received auto-HSCT
- Of the five patients with PR who underwent auto-HSCT, four achieved CR following auto-HSCT
Survival
- After a median follow-up of 20.7 months, the median progression-free survival and overall survival have not been reached.
- The 12-month progression-free survival and overall survival rates were 96.6% and 100%, respectively.
Safety
Grade 3 treatment-emergent adverse events occurred in 28.6% of patients (Table 1).
Table 1. TEAEs occurring in ≥5% of patients*
|
TEAEs, % |
Any grade |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|
≥1 TEAE |
97.6 |
16.7 |
50.0 |
28.6 |
2.4 |
|
ALT increased |
57.1 |
52.4 |
2.4 |
2.4 |
0 |
|
Neutrophil count decreased |
47.6 |
14.3 |
19.0 |
14.3 |
0 |
|
Vomiting |
45.2 |
23.8 |
19.0 |
2.4 |
0 |
|
Nausea |
42.9 |
40.5 |
2.4 |
0 |
0 |
|
White blood cell decreased |
38.1 |
9.5 |
21.4 |
4.8 |
2.4 |
|
AST increased |
35.7 |
31.0 |
4.8 |
0 |
0 |
|
RCCEP |
35.7 |
35.7 |
0 |
0 |
0 |
|
Hypertriglyceridemia |
35.7 |
31.0 |
2.4 |
2.4 |
0 |
|
Platelet count decreased |
35.7 |
26.2 |
4.8 |
4.8 |
0 |
|
Hyperuricemia |
14.3 |
14.3 |
0 |
0 |
0 |
|
LDH increased |
11.9 |
11.9 |
0 |
0 |
0 |
|
Anemia |
11.9 |
11.9 |
0 |
0 |
0 |
|
Fever |
9.5 |
9.5 |
0 |
0 |
0 |
|
Infectious pneumonia |
9.5 |
0 |
9.5 |
0 |
0 |
|
Interstitial pneumonia |
9.5 |
0 |
4.8 |
4.8 |
0 |
|
Pruritus |
9.5 |
7.1 |
2.4 |
0 |
0 |
|
Anorexia |
9.5 |
9.5 |
0 |
0 |
0 |
|
Hypokalemia |
7.1 |
7.1 |
0 |
0 |
0 |
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; RCCEP, reactive cutaneous capillary endothelial proliferation; TEAE, treatment-emergent adverse events. |
|||||
| Key learnings |
| These results indicate that camrelizumab in combination with GEMOX is an effective salvage therapy with a tolerable safety profile in patients with R/R cHL, allowing patients to proceed to auto-HSCT consolidation. |
- Liu Y, Ping L, Song Y, et al. Camrelizumab plus gemcitabine and oxaliplatin for relapsed or refractory classical Hodgkin lymphoma: a phase II trial. BMC Med. 2024;22(1):107. DOI: 1186/s12916-024-03329-8

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








