All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
An expert panel hosted by

Sequencing immune-based therapies in B-cell malignancies
with Ulric Jäger, Sagar Lonial, and Krina Patel

Saturday, June 15 | 18:00-19:30 CEST
Register nowThis independent education activity is sponsored by Bristol Myers Squibb. All content is developed independently by the faculty. Funders are allowed no direct influence on the content of this activity.
The Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
CAR T-cell therapy in LBCL: Key updates from ASH 2023
By Sabina Ray
Bookmark this article
CD19-directed chimeric antigen receptor (CAR) T-cell therapies have shown high efficacy and complete remission (CR) rates in both early phase and subsequent multicenter trials of patients with relapsed/refractory (R/R) B-cell malignancies.
During the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, updates from four key trials of CAR T-cell treatment developments for patients with R/R diffuse large B-cell lymphoma were presented. Here, we summarize these updates; Sehgal presented an updated analysis on the efficacy of lisocabtagene maraleucel (liso-cel),1 Iacoboni presented a retrospective analysis of CAR-T cell therapy post-bispecific antibody treatment,2 Tun presented real-world outcomes of anti-CD19 therapy,3 and Spiegel presented the 5-year follow-up results of axicabtagene ciloleucel (axi-cel).4
Lisocabtagene maraleucel1
PILOT (NCT03483103) is an open-label, randomized, phase II trial investigating liso-cel in patients aged ≥18 years with R/R diffuse large B-cell lymphoma who were not intended for hematopoietic stem cell transplantation after one prior line of therapy. The primary endpoint was met in the primary analysis, with an overall response rate (ORR) of 80%. Key secondary endpoints included CR rate, duration of response, progression-free survival (PFS), overall survival (OS), and safety.
A total of 61 patients were included in the analysis. After a median follow-up of 18.2 months:
- ORR was 80% and 54% achieved CR
- Median duration of response was 23.3 months (95% confidence interval [CI], 6.2–not reached [NR]) for all patients and NR for patients achieving CR
- Median PFS was 9 months (95% CI, 4.2–NR) for all patients and NR for patients achieving CR
- Median OS was NR (95% CI, 16.3–NR)
Safety was assessed during the treatment-emergent period (analysis ≤90 days after liso-cel treatment) and after (analysis started from 91 days posttreatment). More treatment-emergent Grade ≥3 adverse events were reported during the treatment-emergent period compared with after (Table 1). There were a total of 24 deaths reported in the study, most were due to disease progression with the majority occurring more than 90 days after infusion.
Table 1. Grade ≥3 AEs reported during and post the treatment-emergent period*
|
Adverse events, % |
During treatment-emergent period |
After treatment-emergent period |
|
Any Grade ≥3 |
79 |
18 |
|
Neutropenia |
48 |
2 |
|
Anemia |
11 |
5 |
|
Thrombocytopenia |
21 |
5 |
|
AE, adverse event. |
||
Presenter’s conclusions
This follow-up analysis demonstrated that liso-cel is an effective treatment with a manageable safety profile in patients with R/R large B-cell lymphoma (LBCL) for whom hemopoietic stem cell transplantation was not intended, supporting this treatment as a second-line therapy option for this patient cohort.1
T-cell exhaustion post bispecific antibody treatment2
This retrospective, international study investigated the efficacy and safety of anti-CD19 CAR T-cell therapy post bispecific antibody (BsAb) treatment in patients with R/R LBCL. Initially, 47 patients were analyzed from 15 centers in France and Spain who received CAR T-cell therapy (axi-cel, tisagenlecleucel [tisa-cel] and liso-cel) after previous treatment with BsAb. Survival outcomes were comparable before and after CAR T-cell treatment (Table 2).
Table 2. Comparing efficacy and safety in patients post BsAb treatment but pre CAR T-cell treatment to outcomes after both treatments*
|
Result, % (unless otherwise specified) |
After BsAb treatment but before CAR T-cell treatment |
After BsAb treatment and CAR T-cell treatment |
|
Efficacy |
||
|
Best ORR |
47 |
85 |
|
Best CR |
19 |
43 |
|
Median PFS, months |
3.1 |
6.6 |
|
Grade ≥3 AE |
||
|
ICANS |
0 |
2 |
|
CRS |
2 |
6 |
|
AE, adverse event; BsAb, bispecific antibody; CAR, chimeric antigen receptor; CR, complete response; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ORR, overall response rate; PFS, progression-free survival. |
||
Next, a total of 84 patients were assessed in the matched analysis from the DESCAR-T registry (n = 42 with previous BsAb; n = 42 without). Patients with previous BsAb treatment vs without previous BsAb treatment had:
- a higher ORR (86% vs 55% respectively);
- similar complete remission between both cohorts (43% vs 38%, respectively; p = 0.02); and
- 1-year PFS and OS were also similar between patients with previous BsAb treatment vs those without (43% vs 29%, respectively; 55% vs 37%, respectively).
There were no differences in toxicity between patients previously treated with BsAb and those who had not (Table 3).
Table 3. Toxicity in patients treated with previous BsAb treatment vs not*
|
Toxicity, % |
Previous BsAb treatment |
No previous BsAb treatment |
|
CRS, any |
86 |
76 |
|
Grade ≥2 |
38 |
40 |
|
Grade ≥3 |
7 |
19 |
|
ICANS, any |
26 |
26 |
|
Grade ≥2 |
17 |
19 |
|
Grade 3 |
2 |
14 |
|
BsAb, bispecific antibody, CRS, cytokine release syndrome; ICANS, immune effector-cell associated neurotoxicity syndrome. |
||
Presenter’s conclusions
CAR T-cell therapy survival and response rates post-BsAb treatment were similar to patients without previous BsAb treatment and the safety profile was in line with reports from other studies.2
Real-world outcomes of axi-cel, liso-cel, and tisa-cel in R/R LBCL3
This multicenter retrospective study evaluated the safety and efficacy of CAR T-cell therapy in patients aged ≥65 years with R/R LBCL. Primary endpoints included PFS, OS, ORR, and CR and were assessed in 226 patients across seven US centers.
A follow-up of 18.3 months across the population showed:
- median PFS was 6.9 months;
- median OS was 19.1 months;
- ORR was 83% 30 days after treatment and best ORR was 86% after follow-up; and
- CR was 50% after 30 days of treatment and best CR was 62% after follow-up.
Grade ≥3 cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were impacted by Eastern Cooperative Oncology Group performance status, lactate dehydrogenase, and the type of CAR T-cell therapy, but not impacted by age (Table 4). In total, 7% of patients reported Grade 3–4 CRS and 26% reported Grade 3–4 ICANS. For the management of CRS/ICANS, 56% received tocilizumab and 47% were treated with corticosteroids.
Table 4. Characteristics of patients and percentage of reported Grade ≥3 CRS and ICANS.*
|
Characteristic |
N |
Grade ≥3 CRS, % |
p value |
Grade ≥3 ICANS, % |
p value |
|
Age |
|||||
|
≥65–69 |
89 |
4 |
0.49 |
20 |
0.37 |
|
≥70–74 |
63 |
11 |
29 |
||
|
≥75–79 |
47 |
6 |
30 |
||
|
≥80–89 |
26 |
8 |
35 |
||
|
ECOG PS score |
|||||
|
≤2 |
186 |
6 |
0.77 |
23 |
0.007 |
|
≥2 |
27 |
7 |
48 |
||
|
LDH prior to CAR T-cell therapy |
|||||
|
Normal |
104 |
4 |
0.18 |
16 |
0.003 |
|
Elevated |
110 |
8 |
34 |
||
|
Type of CAR T-cell therapy |
|||||
|
Axi-cel |
131 |
8 |
0.35 |
34 |
0.002 |
|
Tisa-cel |
37 |
11 |
11 |
||
|
Liso-cel |
57 |
4 |
19 |
||
|
axi-cel, axicabtagene ciloleucel; CAR T, chimeric antigen T-cell therapy; CRS, cytokine release syndrome; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICANS, immune effector cell-associated neurotoxicity syndrome; LDH, lactate dehydrogenase; liso-cel, lisocabtagene maraleucel; tisa-cel, tisagenlecleucel. |
|||||
Presenter’s conclusions
This real-world evidence study showed that axi-cel, liso-cel, or tisa-cel treatment had promising efficacy and a safety profile comparable to pivotal clinical trials in older patients (≥65 years) with R/R LBCL. As such, elderly patients should not be excluded from CAR T-cell treatment solely based on age.3
Axi-cel analysis 5 years posttreatment4
Spiegel presented a real-world evidence study with follow-up data 58 months post axi-cel treatment in patients with R/R LBCL. Primary endpoints were OS and PFS evaluated in 275 patients. At follow-up:
- The 5-year OS and PFS were 40.3% and 28.5%, respectively; these results were similar to the ZUMA-1 trial
- Day 30 ORR was 80% and CR rate was 44%, which was similar to the best ORR and CR reported (82% and 64%, respectively)
- Approximately 29% of patients remain in remission, including 43% of patients who were ineligible for the ZUMA-1 trial.
- Similar to the results from Tun.3 described above, lactate dehydrogenase above the upper limit of normal and an ECOG Performance Status 2–4 were associated with lower OS and PFS compared with patients who did not, as well as elevated bilirubin and male sex. Interestingly, age did impact PFS in this study
The top two causes of death over the 5-year investigation included progressive disease (n = 118), and infection (n = 21; Figure 1). This was followed by secondary malignancy (n = 9), CAR T-cell toxicity (n = 3), or unknown/other (n = 7).
Figure 1. Top causes of death over 5-years*
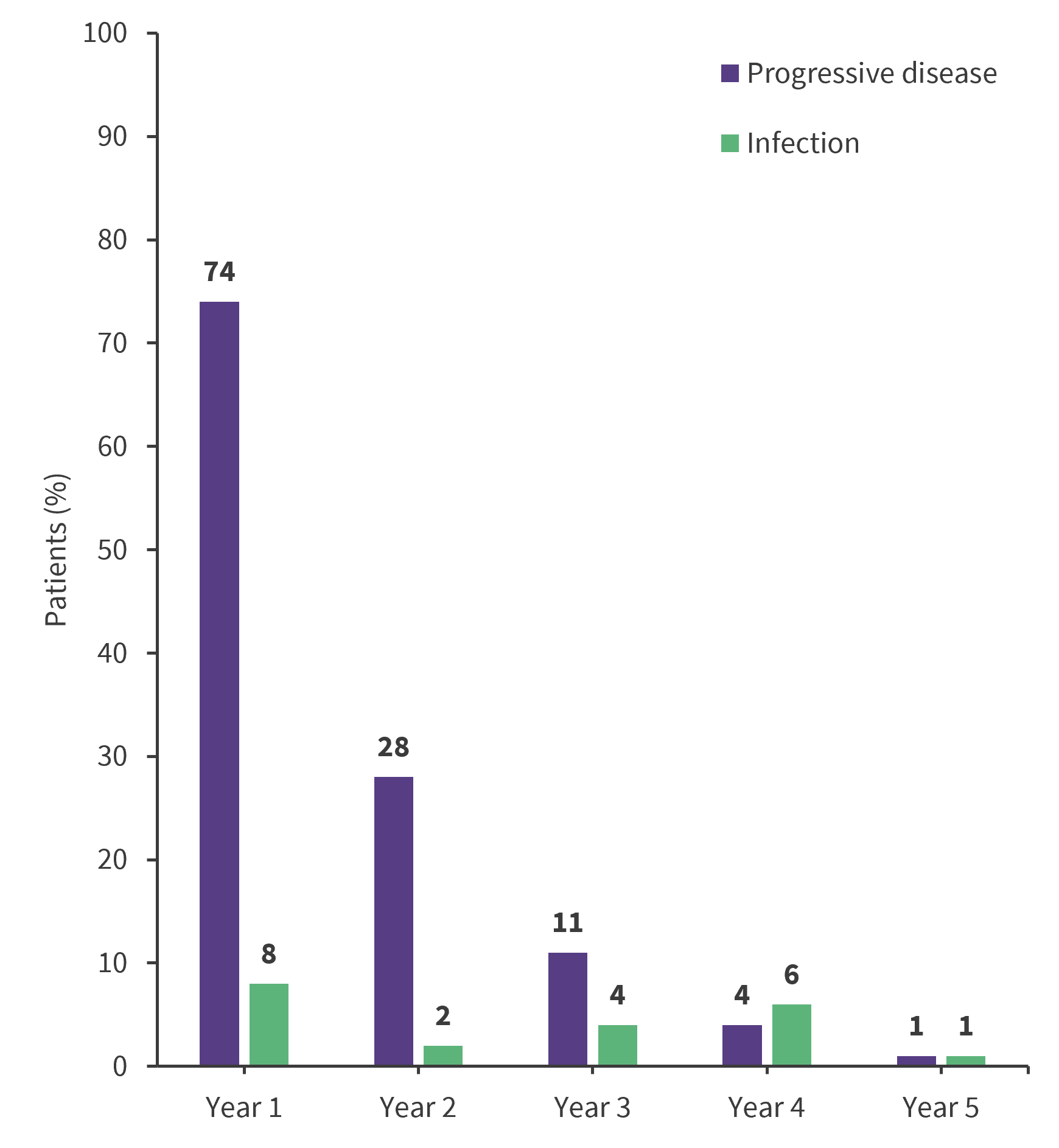
*Adapted from Spiegel.4
Presenter’s conclusions
The efficacy and safety reported were similar to the 5-year follow-up to the ZUMA-1 trial, despite including patients who were not eligible for this study due to comorbidities. This study highlights important survivorship issues after axi-cel treatment. Axi-cel is considered a potential treatment but cautions against the competing risk of non-relapse mortality in a high-risk population.4
- Sehgal AR. Lisocabtagene maraleucel as second-line therapy for R/R large B-cell lymphoma in patients not intended for hematopoietic stem cell transplant: final analysis of the phase 2 PILOT study. Oral abstract #105. 65th American Society of Hematology Annual Meeting and Exposition; Dec 9, 2023; San Diego, US.
- Iacoboni G. Efficacy of chimeric antigen receptor T-cell therapy is not impaired by previous bispecific antibody treatment in patients with large B-cell lymphoma. Oral abstract #228. 65th American Society of Hematology Annual Meeting and Exposition; Dec 9, 2023; San Diego, US.
- Tun AM. Chimeric antigen receptor T-cell therapy in elderly patients with relapsed or refractory large B-cell lymphoma: a multicenter study. Oral abstract #311. 65th American Society of Hematology Annual Meeting and Exposition; Dec 9, 2023; San Diego, US.
- Spiegel JY. Five year outcomes of patients with large B-cell lymphoma treated with standard-of-care axicabtagene ciloleucel: results from the US lymphoma CAR-T cell consortium. Oral abstract #1032. 65th American Society of Hematology Annual Meeting and Exposition; Dec 11, 2023; San Diego, US.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








