All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
DLBCL at high risk of recurrence after R-CHOP: no improvement in DFS with enzastaurin versus placebo
Bookmark this article
The results of the PRELUDE study (NCT00332202 trial) were published by M. Crump from the Princess Margaret Cancer Center in Toronto, and colleagues in J Clin Oncol. in July 2016. Unfortunately, the results of this trial were not as hopeful as expected.
The primary objective of this study was to compare Disease-Free Survival (DFS) with enzastaurin as maintenance therapy versus placebo in patients with Diffuse Large B-Cell Lymphoma (DLBCL) who were at a high risk of experiencing relapse after achieving a Complete Response (CR) with rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as first-line therapy. Biomarker analysis was a secondary study objective.
Here are the take home messages:
- Enzastaurin, when administered for 3 years as maintenance therapy, to patients who have achieved CR after first-line R-CHOP therapy, did not significantly improve DFS compared with placebo
- Identifying the value of specific biomarkers in predicting therapeutic response to novel targeted agents may be useful for future trials within defined subgroups of patients with DLBCL
Randomized, Double-Blind, Phase III Trial of Enzastaurin versus Placebo in Patients Achieving Remission after First-Line Therapy for High-Risk Diffuse Large B-Cell Lymphoma
Abstract
Purpose: To compare disease-free survival (DFS) after maintenance therapy with the selective protein kinase C β (PKCβ) inhibitor, enzastaurin, versus placebo in patients with diffuse large B-cell lymphoma (DLBCL) in complete remission and with a high risk of relapse after first-line therapy.
Patients and Methods: This multicenter, phase III, randomized, double-blind, placebo-controlled trial enrolled patients who were at high risk of recurrence after rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Patients (N = 758) with stage II bulky or stage III to IV DLBCL, three or more International Prognostic Index risk factors at diagnosis, and a complete response or unconfirmed complete response after 6 to 8 cycles of R-CHOP were assigned 2:1 to receive oral enzastaurin 500 mg daily or placebo for 3 years or until disease progression or unacceptable toxicity. Primary end point was DFS 3 years after the last patient entered treatment. Correlative analyses of biomarkers, including cell of origin by immunohistochemistry and PKCβ expression, with efficacy outcomes were exploratory objectives.
Results: After a median follow-up of 48 months, DFS hazard ratio for enzastaurin versus placebo was 0.92 (95% CI, 0.689 to 1.216; two-sided log-rank P = .541; 4-year DFS, 70% v 71%, respectively). Independent of treatment, no significant associations were observed between PKCβ protein expression or cell of origin and DFS or overall survival.
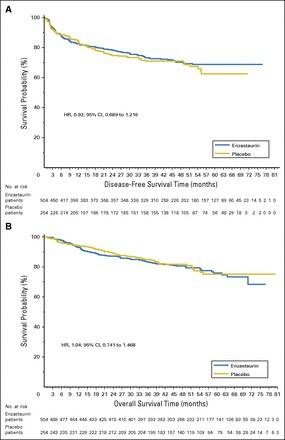
Conclusion: Enzastaurin did not significantly improve DFS in patients with high-risk DLBCL after achieving complete response to R-CHOP. Achievement of a complete response may have abrogated the prognostic significance of cell of origin by immunohistochemistry.
Footnotes
- Supported by Eli Lilly
- Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article
- Clinical trial information: NCT00332202
- M. Crump et al. Randomized, Double- Blind, Phase III Trial of Enzastaurin versus Placebo in Patients Achieving Remission after First-Line Therapy for High-Risk Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2016 Jul 20; 34(21): 2484-92. doi: 10.1200/JCO.2015.65.7171. Epub 2016 May 23.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








