All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Epidemiology, pathology, and clinical features of DLBCL
Bookmark this article
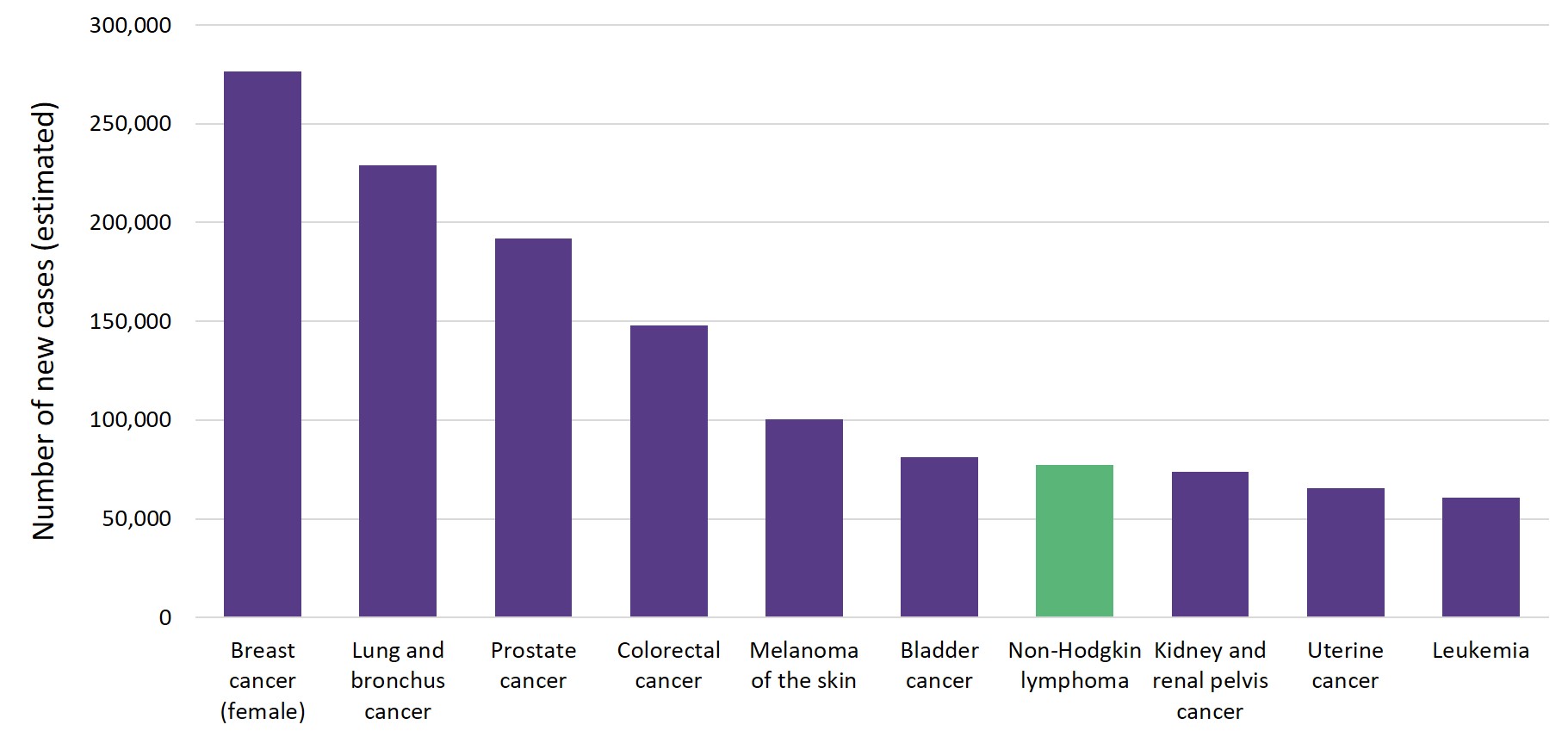
Non-Hodgkin lymphoma (NHL) has an annual incidence of ~ 16–24 cases per 100,000 persons in the United States and accounts for an estimated 4.3% of all newly diagnosed cancers.1 Figure 1. shows the number of estimated new cases of NHL in 2020 in comparison to other common cancers in the U.S. The prevalence of NHL increases with age, from 0.05% and 0.03% before the age of 40 to 1.30% and 0.96% after the age of 75, in men and women, respectively.1
Diffuse large B-cell lymphoma (DLBCL) is the most common type of NHL, accounting for approximately 31% of all NHL in Western countries and 37% of B-cell tumors worldwide.2 The median age at presentation is 66, however, DLBCL can occur at any age. There is a slightly higher incidence in men (6.7 vs 4.6 cases per 100,000 persons, male vs female).1 The probability of having DLBCL increases with age, from 0.13% and 0.09% before the age of 39 to 1.77% and 1.4% after the age of 70 in men and women, respectively.3 5-year survival rates decrease by age from 78% for those aged < 55 years to 54% for those aged ≥ 65 years.1
Figure 1. Number of new cancer cases (estimated) in the U.S. in 20201
The clinical presentation of DLBCL is diverse and depends on the site of disease involvement. Extranodal involvement is common and is seen in up to one-third of patients at the time of diagnosis.4 The most common extranodal presentation is in the gastrointestinal tract, followed by the bone, testis, spleen, salivary gland, thyroid, liver, kidney, and adrenal gland. DLBCL is an aggressive disease, with tumors showing a rapid growth rate and patients presenting with large masses that infiltrate tissues or obstruct organs. Patients often present with B symptoms, including fever, night sweats, and weight loss.
DLBCL is clinically, morphologically, and genetically a highly heterogeneous lymphoid malignancy. It can arise from mature B cells at different stages of differentiation. Gene mutations promote changes in B cells, thus changing their gene expression and promoting neoplastic transformation. These lymphoid cells have a nuclear size equal to or exceeding normal macrophage nuclei or more than twice the size of a normal lymphocyte.5 Just some of the many biomarkers identified (but not always expressed) in the malignant B-cells include CD19, CD79a, CD20, PAX5, and CD22; other markers that may be expressed with variable frequency include CD38 and CD138, CD30 (associated with anaplastic morphology), and CD5.6 DLBCL may arise as primary or de novo, or may result from the progression or transformation of other B-cell neoplasms such as chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, marginal zone lymphoma, follicular lymphoma, and lymphocyte-predominant Hodgkin lymphoma.2
Morphological, biological, and clinical studies have allowed the subdivision of DLBCL into morphological variants, molecular and immunophenotypic subgroups, and distinct disease entities. However, a large number of cases still remain biologically heterogeneous for which there are no clear and accepted criteria for subclassification: these are collectively termed DLBCL not otherwise specified (NOS).2
According to the 2016 World Health Organization (WHO) classification, DLBCL can be subdivided into morphological and immunological variants:
- Centroblastic variant: a predominant population of medium to large lymphoid cells with oval to round nuclei
- Immunoblastic variant: > 90% immunoblasts and large cells with large nuclei
- Anaplastic variant: large to very large cells and pleomorphic nuclei
Other variants include primary cutaneous DLBCL, Epstein-Barr virus positive DLBCL, and primary central nervous system DLBCL.5 Additionally, subgroups of DLBCL can be classified based on gene expression profiling, including the germinal center B-cell (GCB) and the activated B-cell (ABC) types, which are associated with specific genetic alterations, different molecular pathways and clinical outcomes.7 For more information about the most common epigenetic modifiers in GCB and ABC DLBCL, read our article here.
Further subgroups included in the WHO classification that do not strictly fit into the GCB or ABC subtypes include those with high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 translocations, termed double-/triple-hit lymphomas, respectively.7 These mutations confer a prognostic relevance as patients with double-/triple-hit mutations often demonstrate inferior outcomes to standard chemotherapy (rituximab, cyclophosphamide, vincristine doxorubicin, and prednisolone; R-CHOP).8
More recently, a number of gene expression profiling studies using various tools, including consensus clustering, array-based DNA copy-number analysis, and targeted amplicon resequencing, have allowed for the separation of DLBCL into multiple classifications with distinct pathogenic mechanisms and outcomes.9,10 In 2020, Wright et al. published an article in the journal Cancer Cell, describing an algorithm which can determine the probability that a patient’s lymphoma belongs to one of seven genetic subtypes based on its genetic features and highlights the distinct vulnerabilities to targeted therapy for each group (Table 1).11
Table 1. DLBCL classifications based on genetic subtypes (adapted from Wright et al.11)
|
BCL2, B-cell lymphoma 2; BCLXL, B-cell lymphoma–extra large; BCR, B-cell receptor; EZH2, enhancer of zeste homolog 2; GNA13, G protein subunit alpha 13; IgG, immunoglobulin G; IgM, immunoglobulin M; IRAK4, interleukin 1 receptor associated kinase 4; IRF4, interferon regulatory factor 4; JAK, Janus kinase; MCL1, myeloid cell leukemia 1; MHC, major histocompatibility complex; mTORC1, mammalian target of rapamycin complex 1; NF-κB, nuclear factor-kappa B; PI3K, phosphoinositide 3-kinase; P2RY8, purinergic receptor P2Y8; STAT, signal transducer and activator of transcription; S1PR2, Sphingosine-1-phosphate receptor 2; TP53, tumor suppressor p53; TFH, T follicular helper cells. |
|||
|
Genetic subtype |
Prevalence (%) |
Genetic themes |
Potential therapeutic targets |
|---|---|---|---|
|
MCD |
8.7 |
MY-T-BCR-dependent NF-κB |
BCR-dependent NF-κB, PI3K, mTORC1, BCL2-BCLXL-MCL1, JAK1, IRAK4, IRF4 |
|
N1 |
1.7 |
NOTCH1 signaling |
NOTCH1, immune checkpoints |
|
A53 |
58 |
TP53 inactivation/DNA damage |
BCR-dependent NF-κB, |
|
BN2 |
13.3 |
NOTCH2 signaling |
BCR-dependent NF-κB, PI3K, mTORC1, BCL2, NOTCH2 |
|
ST2 |
6.4 |
JAK/STAT signaling |
PI3K, JAK2 |
|
MYC+ |
5.9 |
Chromatin modification |
PI3K, mTORC1, EZH2, BCL2-MCL1 |
|
MYC– |
17.6 |
||
DLBCL is heterogeneous at a molecular, clinical, and morphological level. With the advancement of newer technologies, and further insights into this heterogeneity, there is hope for more personalized treatment strategies for these different DLBCL subgroups.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. seer.cancer.gov. Released Apr 2020, based on the Nov 2019 submission (1975-2017). Accessed Jan 18, 2021.
- Martelli M, Ferreri AJM, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146-71. DOI: 10.1016/j.critrevonc.2012.12.009
- Sarkozy C, Coiffier B. Diffuse large B-cell lymphoma in the elderly: A review of potential difficulties. Clin Cancer Res. 2013;19(7):1660-1669. DOI: 1158/1078-0432.CCR-12-2837
- López-Guillermo A, Colomo L, Jiménez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23(12):2797-2804. DOI: 1200/JCO.2005.07.155
- Gouveia GR, Siqueira SAC, Pereira J. Pathophysiology and molecular aspects of diffuse large B-cell lymphoma. Rev Bras Hematol Hemoter. 2012;34(6):447-451. DOI: 5581/1516-8484.20120111
- Gurbaxani S, Anastasi J, Hyjek E. Diffuse large B-cell lymphoma—more than a diffuse collection of large B cells: an entity in search of a meaningful classification. Arch Pathol Lab Med. 2009;133(7):1121-1134. DOI: 1043/1543-2165-133.7.1121
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. DOI: 1182/blood-2016-01-643569
- Davies A. Double-hit lymphoma: So what? Hematol Oncol. 2019;37(suppl 1):19-23. DOI: 1002/hon.2581
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679-690. DOI: 1038/s41591-018-0016-8
- Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396-1407. DOI: 10.1056/NEJMoa1801445
- Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551-568.e14. DOI: 1016/j.ccell.2020.03.015

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








