All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
EHA-SWG 2017 | Rare Lymphomas: T-Cell Lymphoma novel treatment options
Bookmark this article
On March 10–12 2017, the EHA-SWG meeting on Rare Lymphomas took place in Barcelona, Spain, and was jointly chaired by Prof. Martin Dreyling, from Klinikum der Universität München, Germany, and Prof. Marie José Kersten, from the Academic Medical Center, Amsterdam, The Netherlands.
On March 12th 2017, Francesco d’Amore, MD, PhD, from Aarhus University Hospital, Denmark, gave a talk discussing novel treatment options for T-Cell Lymphomas.
Intensive Chemotherapy
Firstly, the results of a phase II study of SMILE chemotherapy for newly diagnosed, stage IV, R/R ENKTCL nasal type were discussed: CR = 17 (45%), PR = 13 (34%), 1-yr OS = 55%, 3-yr OS = 50%, 3-yr PFS = 45%.
Treatment of HSTCL treatment was also discussed: induction with ICE/IVAC at the MSKCC resulted in median PFS of 13.3 months and median OS of 59 months. In a different study, 18/25 patients underwent allo-SCT, resulted in a 3-yr PFS of 48%. In those who underwent auto-SCT (n=7), 5 patients relapsed.
Standard approach - CHOP
Francesco D’Amore then stated that the standard approach for PTCL-NOS and AITL, outside of clinical trials, was CHOP or CHOEP/EPOCH with or without ASCT. Outcomes with CHOP were discussed and whether they could be improved.
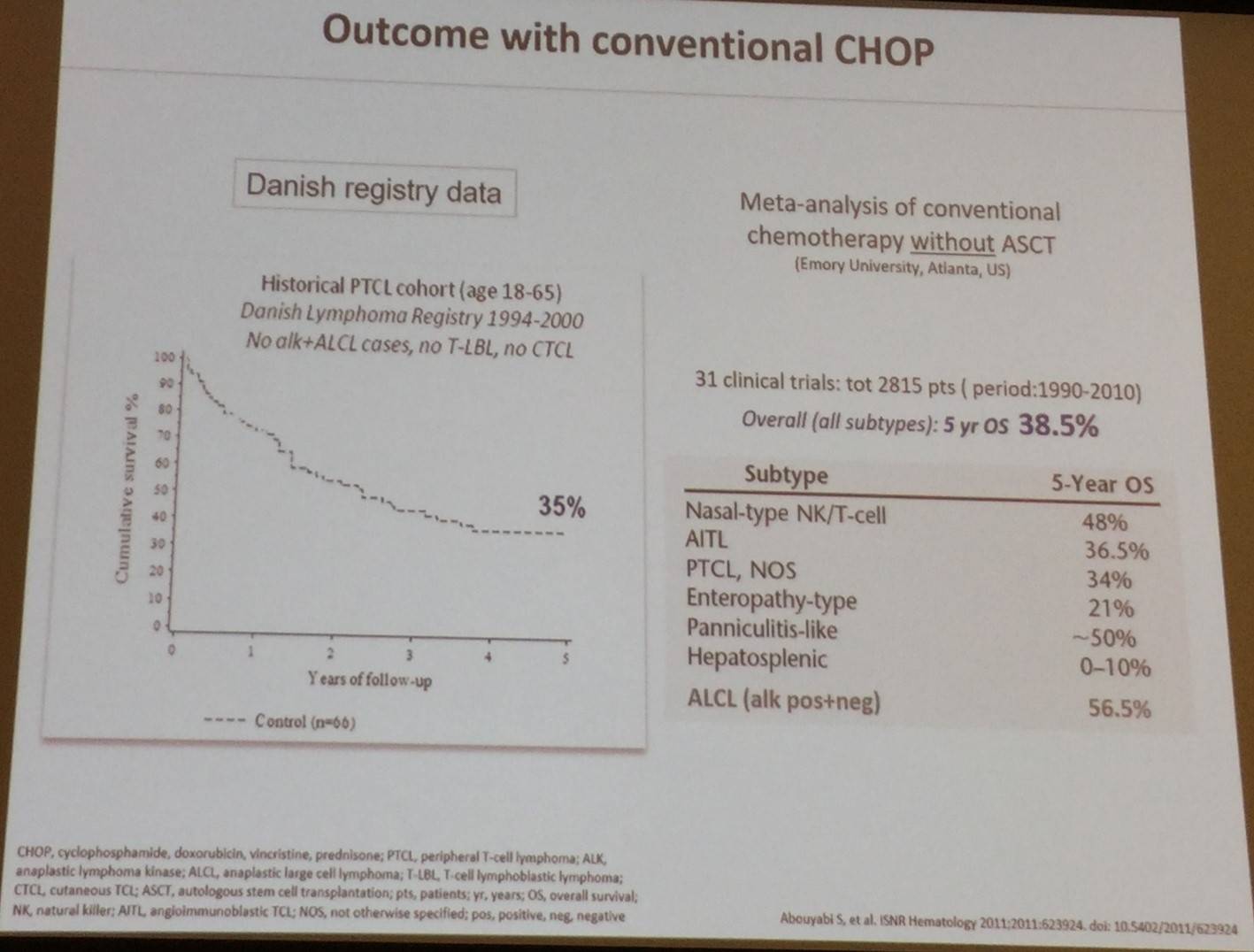
In a phase II study of cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in PTCL (SWOG study S0350), the authors reported “disappointing outcomes”:
- ORR = 39%; CR = 24%; PR = 15%
- Median PFS = 9 months; Median OS = 17 months
- 2-yr PFS = 12%; 2-yr OS = 31% (designed target: 67%)
Moreover, a phase II study of cyclophosphamide, etoposide, vincritine, and prednisone (CEOP) alternating with pralatrexate (P) as first-line treatment for PTCL “did not improve outcomes compared to historical CHOP data” (2-yr PFS = 39%; 2-yr OS = 60%).
Combining CHOP with etoposide
However, when comparing Nordic and German trials, a potential role for etoposide became clear:
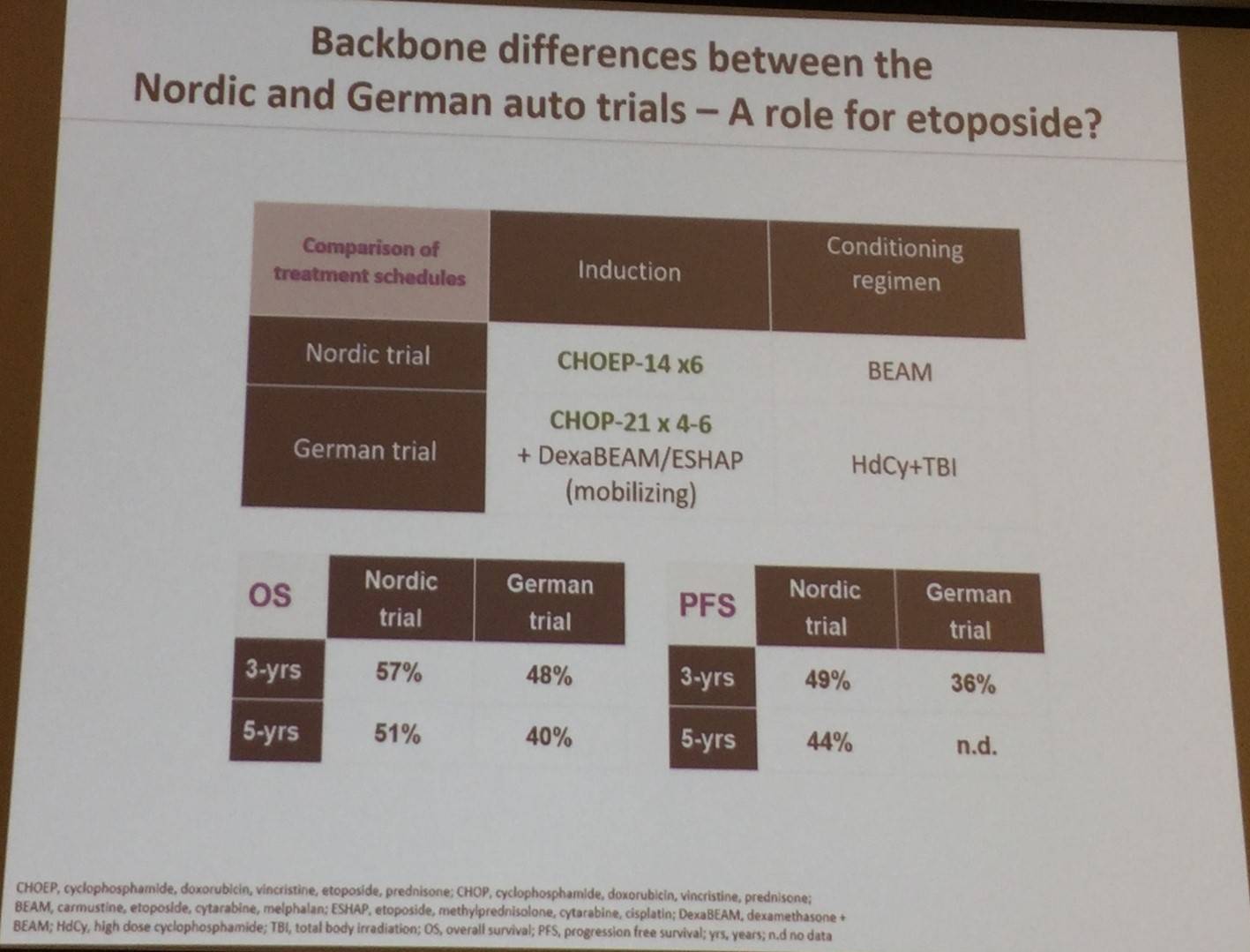
Additionally, “real world” data from a Swedish Lymphoma registry found that in 755 patients with non-leukemic, non-cutaneous PTCL, combining etoposide with CHOP was associated with favorable PFS in younger patients (≤60 years; HR = 0.49; P = 0.008).
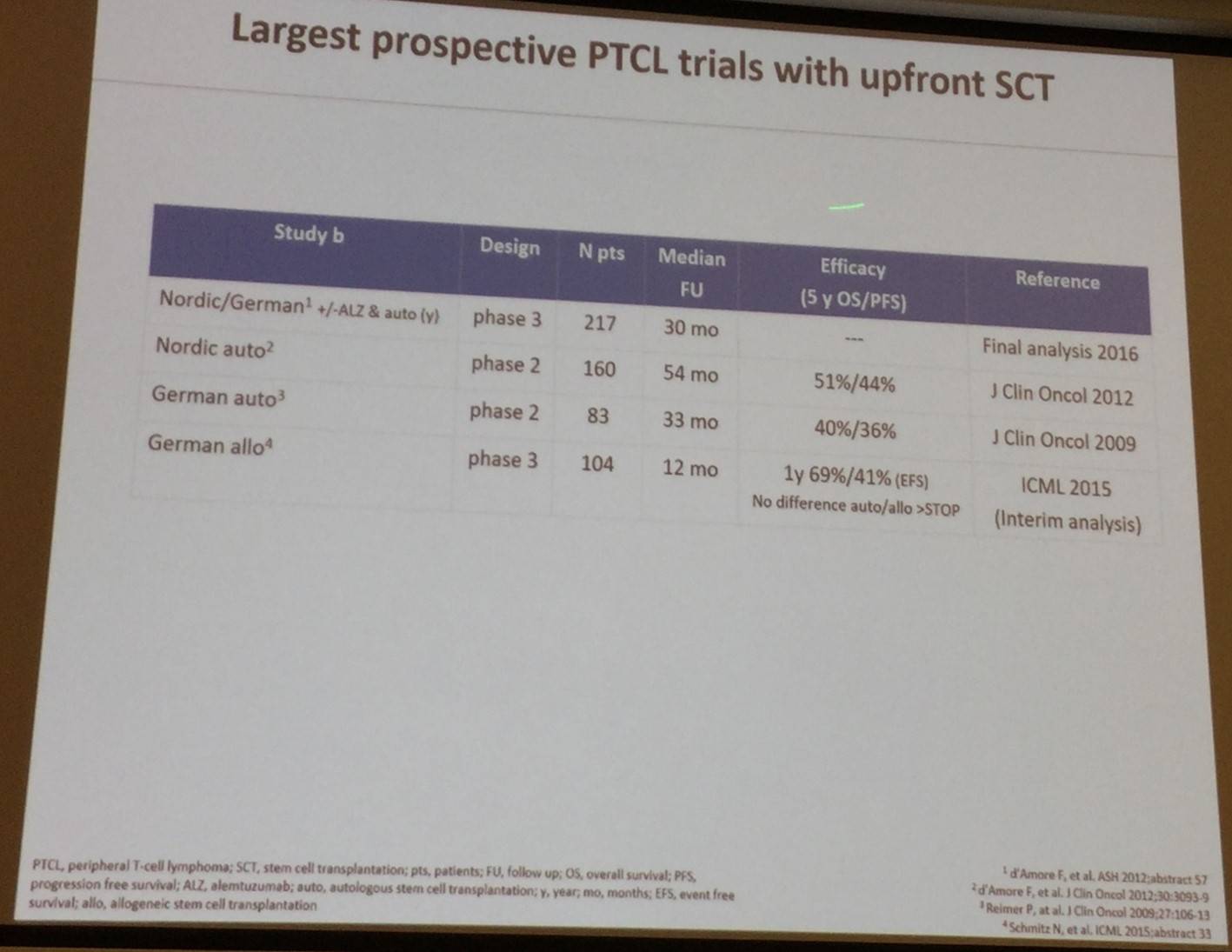
Autologous and allogeneic SCT
Francesco d’Amore then went on to discuss results of the prospective, phase II, NLG-T-01 trial, evaluating HDT and auto-SCT in untreated, systemic PTCL, which were presented at ICML 2015:
- Median follow-up = 10 years (range, 7–13 years)
- Median age at study entry = 57 years
- Overall: OS = 42%; PFS = 38%; DSS = 51%
- ALCL: OS = 48%; PFS = 48%; DSS = 67%
- AILT: OS = 47%; PFS = 40%; DSS = 51%
- PTCL-NOS: OS = 42%; PFS = 38%; DSS = 50%
- EATL: OS = 27%; PFS = 29%; DSS = 30%
The talk also discussed iterim anlaysis results of the AATT trial, comparing allogenic or autologous transplantation as first-line therapy for younger PTCL patients. Main issues were that 38% did not reach consolidative SCT, and GvL-effect of allo was counterbalanced by high TRM. Overally, no significant differences were founf between auto- or allo-SCT (ORR = 53% vs 50%; 1-yr EFS = 48% vs 48%; 1-yr OS = 61% vs 55%).
Results of the largest prospective trials with upfront SCT in PTCL patients were also summarized:
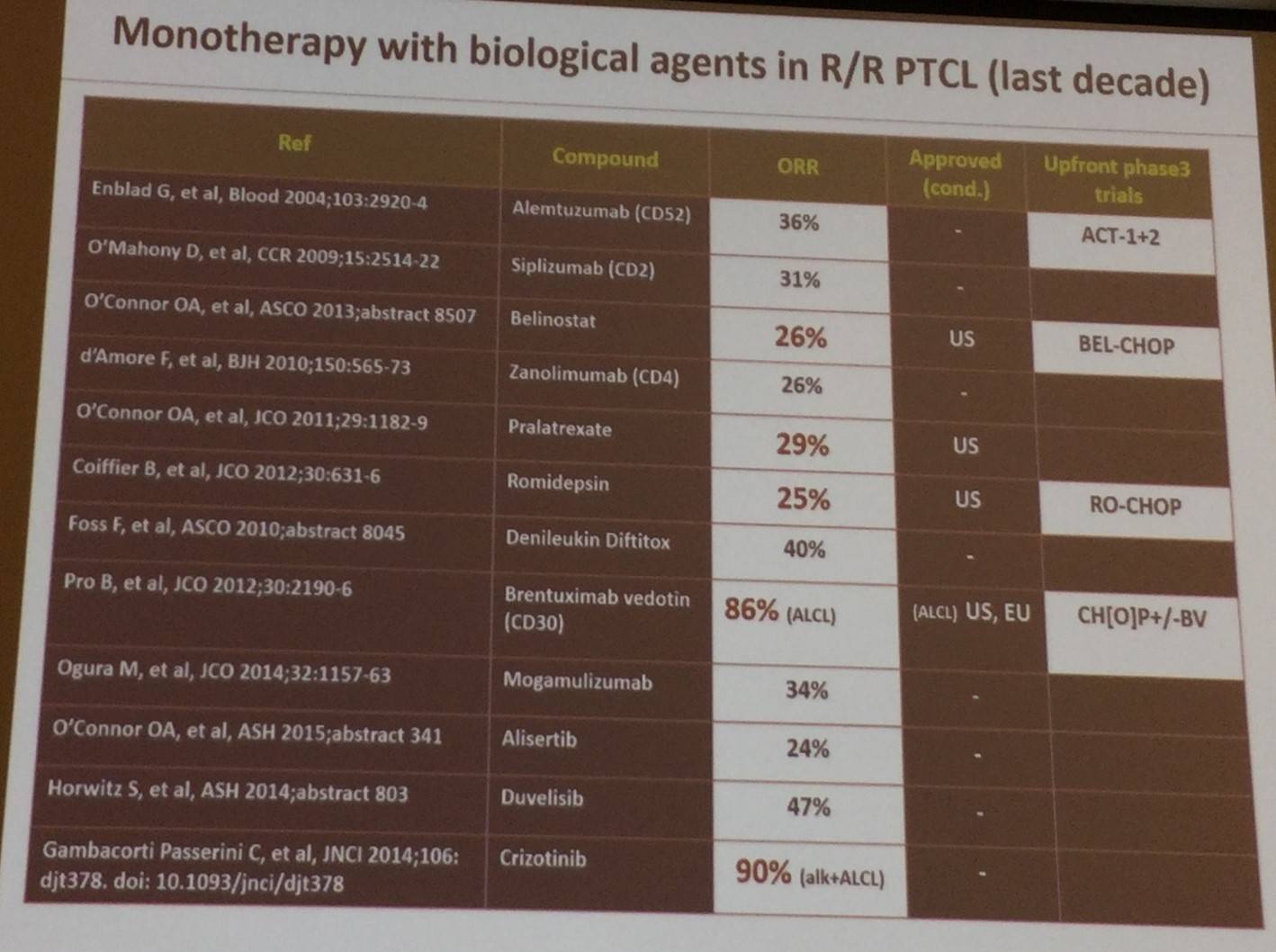
Biological agents for R/R PTCL
ORR results of numerous trials investigating biological agents, such as alemtuxumab, romidepsin, and brentuximab vedotin, in R/R PTCL were shared:
After this, data presented at ICML 2015 of a phase III study which combined romidepsin with CHOP as first-line treatment for PTCL were discussed. Median follow-up was 10 months and 27 patients were evaluable: CR = 15/27 (55.6%); ORR = 20/27 (74%).
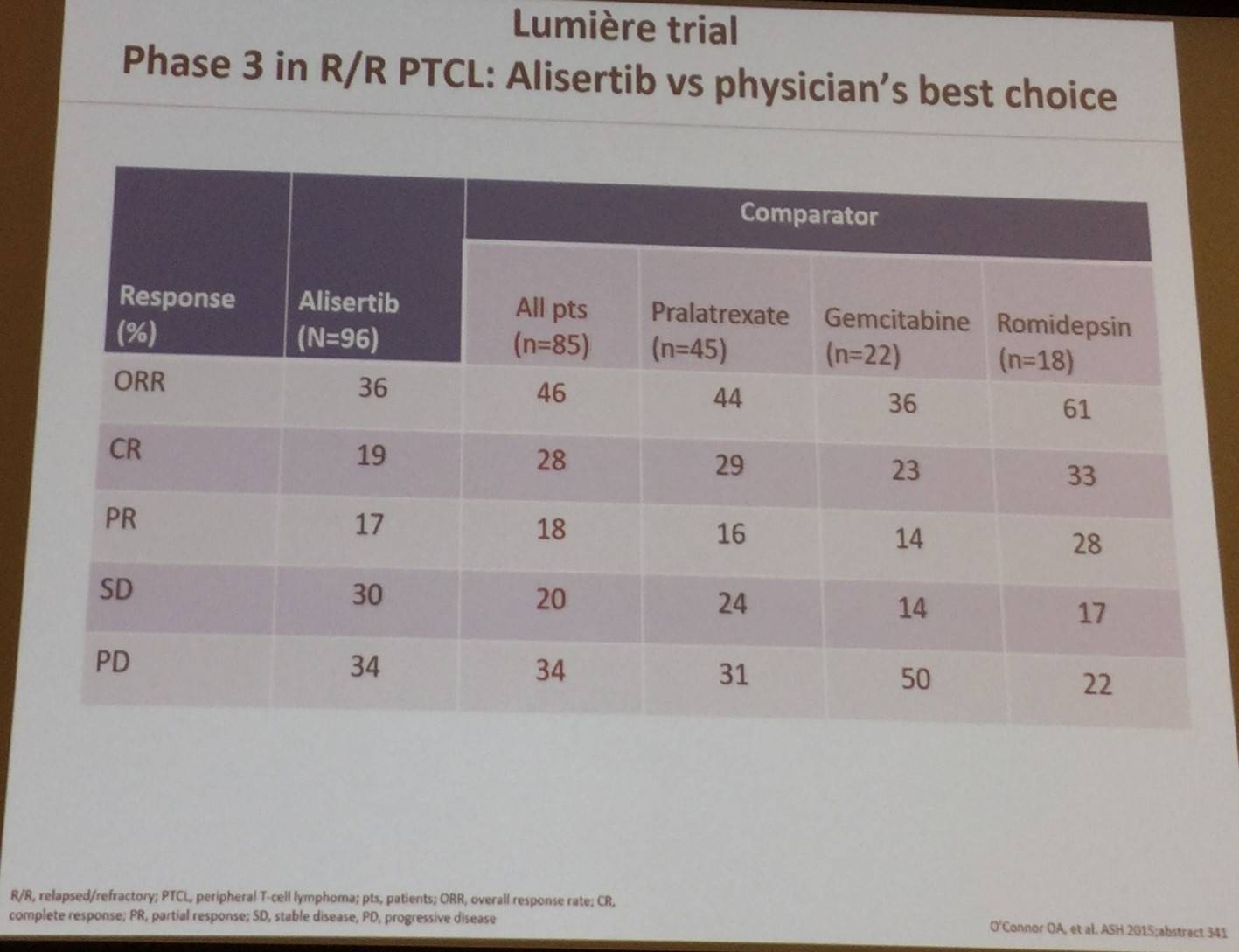
Results of the phase III Lumière trial, investigating alisertib versus physician’s best choice, were also shared:
Mogamulizumab, a defucosylated, humanized, anti-CCR4 antibody, is another option for TCL treatment. The antibody lacks fucose due to knock out of the FUT8 gene, leading to increased ADCC activity. CCR-4 is expressed in approximately 90% of ATLLs, and mogamulizumab is approved in Japan for ATLL therapy. In ATLL: ORR = 50% (CR = 35%); median PFS = 5.2 months. In TCL: ORR = 35% (CR = 13%); median PFS = 3 months.
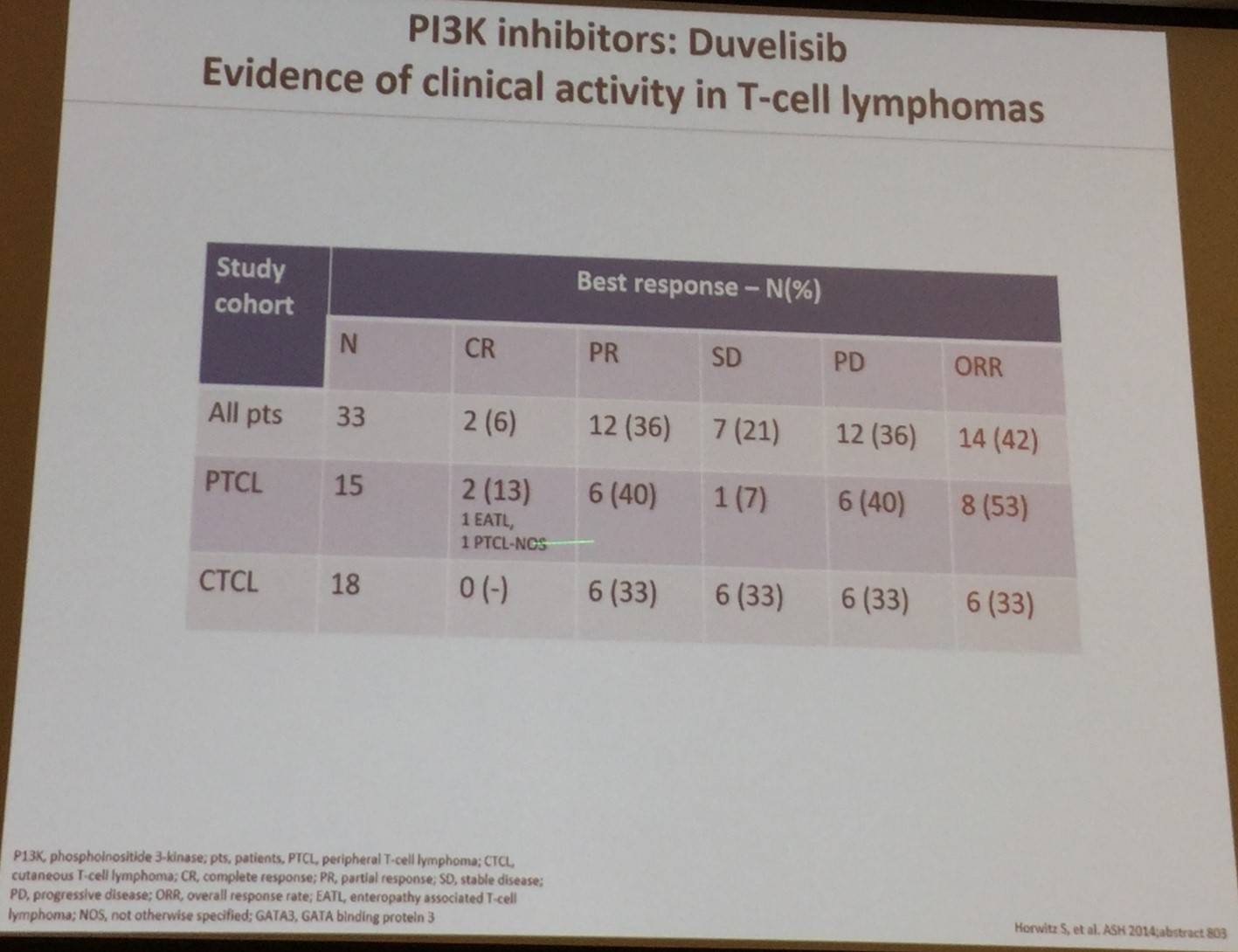
Best response results using the PI3K inhibitor duvelisib in patients with PTCL and CTCL were shared:
Francesco d’Amore also discussed data which indicates that ALK inhibitors appear efficacious in ALK+ ALCL. Crizotinib monotherapy in R/R ALCL+ patients resulted in an ORR of 91% (10/11 patients), and 2-yr OS and 2-yr PFS of 73% and 64%, respectively.
Conclusion
The talk concluded by hypothesizing that by combining information about individual patient’s TCL mutational status and new trials investigating novel agents, we can design regimens which can improve current outcomes.
- d’Amore F. Novel Treatment Options. 2017 Mar 12. EHA-SWG Rare Lymphomas. Barcelona, Spain.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








