All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ESMO 2016 | Educational Session: Lymphomas – New Therapeutic Strategies
Bookmark this article
Andrés J. M. Ferreri from the IRCCS San Raffaele Scientific Institute presented and chaired an educational session focused on emerging therapeutic strategies for patients with Lymphomas at the ESMO congress 2016, at Copenhagen, Denmark.
Advances in clinical research have enabled the emergence of new therapeutic strategies in Lymphomas. Currently, there are more than 800 novel drug agents in clinical development and evidence of activity by some of these agents has been shown in several phase I and II clinical trials. In Lymphomas, there are a number of therapies targeted at signaling pathways involved in cell proliferation, differentiation and survival including PI3K, NFκB, CD40, TNF, BCR and AKT signaling pathways. These targeted therapies aim to either interfere with constitutionally activated signaling pathways, block negative feedback from tumor cells to immune cells, or increase uptake of toxic agents by tumor cells.
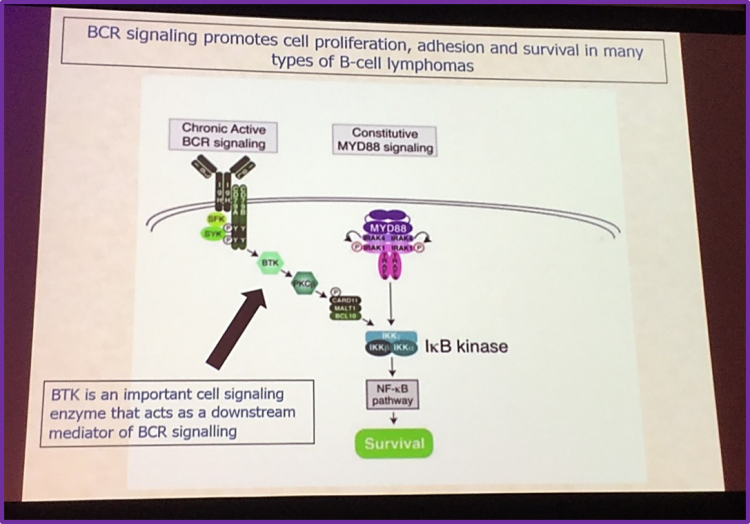
Targeting the B cell receptor (BCR) signaling pathway has proven to be very promising for therapy in Lymphomas. The BCR signaling pathway supports cell survival, proliferation and differentiation in many B-cell Lymphomas. Bruton’s Tyrosine Kinase (BTK) is a kinase located downstream of the BCR signal and it is a crucial mediator in the BCR signaling cascade. Hence, clinical researchers have targeted BTK for therapy in lymphoma patients. Clinical research has shown ibrutinib, a BTK inhibitor, to have a high efficacy in patients with either Relapsed or Refractory (R/R) Mantle Cell Lymphoma (MCL) or in 1st line for Chronic Lymphocytic Leukemia (CLL) / Small Lymphocytic Leukemia and CLL/SLL with 17p deletion and it has been approved by the Food and Drug Agency (FDA) in these indications and by the European Medicines Agency (EMA) in R/R MCL, in 1st line CLL, in combination with rituximab and bendamustine for CLL patients in 2nd line and in Waldenström macroglobulinemia . Additionally, in high-risk patients, data regarding ibrutinib, seem to be encouraging for its use in therapy. Ibrutinib also has a high efficacy in patients with MyD88 Lymphomas and preliminary results indicate that ibrutinib can have an effect in ABC-Diffuse Large B-cell Lymphoma (DLBCL).
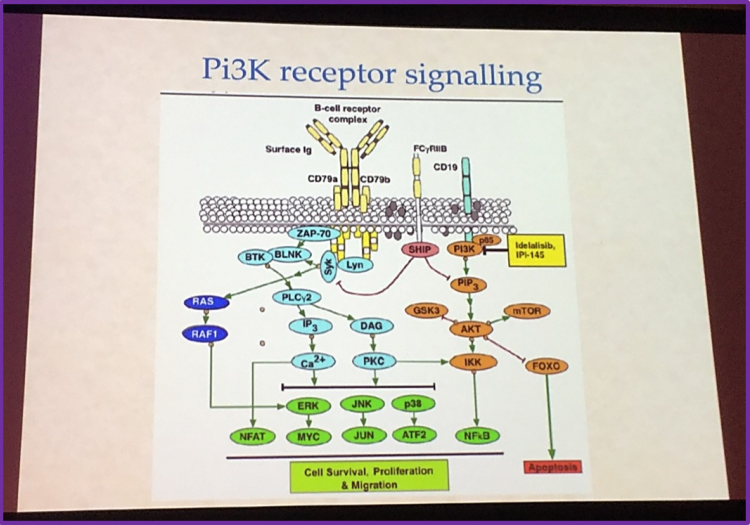
Another strategy researchers have identified for therapy of Lymphomas is the PI3K signaling pathway. The PI3K receptor signaling pathway can signal for cell survival and proliferation in cells and thus a crucial target for therapy in Lymphoma patients. Idelalisib, a PI3K inhibitor, has been shown to be active and effective in R/R Follicular Lymphoma (FL), CLL and MCL. However, there are some adverse side effects associated with this agent including diarrhea, pneumonitis and pyrexia. Idelalisib is currently approved by the FDA for the treatment of patients with either R/R CLL, FL (after 2 prior lines of treatment) or Small Lymphocytic Leukemia (SLL) and approved by the EMA for the treatment of patients with either CLL (in 2nd line or in maintenance for patients with 17p deletion or TP53 mutation; in combination with rituximab) or FL (in monotherapy for R/R patients after 2 lines of therapy). PI3K inhibitors are very promising for therapy in lymphomas and several clinical trials are currently on-going.
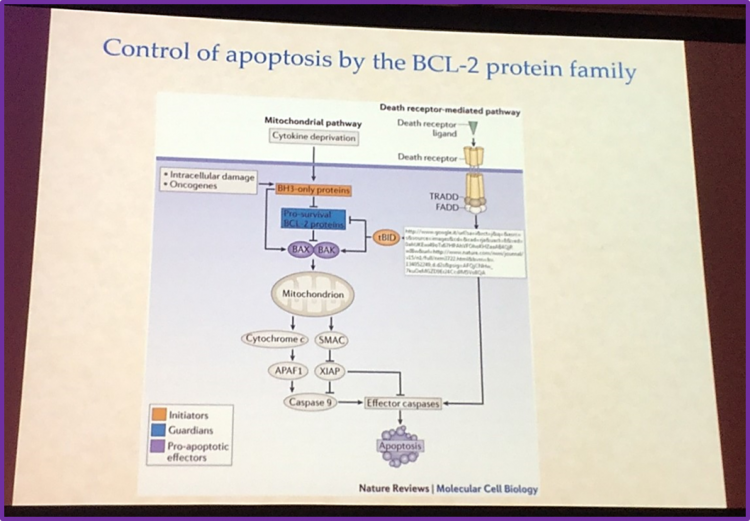
BCL-2 proteins are crucial proteins that regulate apoptotic signaling pathways. BCL-2 proteins can either be pro-survival or pro-apoptotic. A resistance mechanism used by lymphoma cells for survival is when the balance towards pro-survival BCL-2 proteins is gained. Navitoclax, a selective inhibitor of a pro-survival protein called BCL-XL, has shown encouraging clinical efficacy in BCL-2 dependent hematologic cancer. However, it is associated with on target thrombocytopenia, thus limiting this agent for therapy in Lymphoma patients. Clinical researchers re-engineered navitoclax to make venetoclax, which is highly potent in limiting Lymphoma growth but has less severe adverse effects compared to navitoclax. In clinical trials, venetoclax as a single agent has shown anti-tumor activity in a number of Non-Hodgkin Lymphoma (NHL) subtypes and in CLL. In combination therapy, venetoclax either with monoclonal antibodies such as obinutuzumab and rituximab, or chemotherapy agents, in clinical trials has shown promising activity. It would be of interest to use a combination of venetoclax with other targeted therapies such as the BTK inhibitor, ibrutinib.
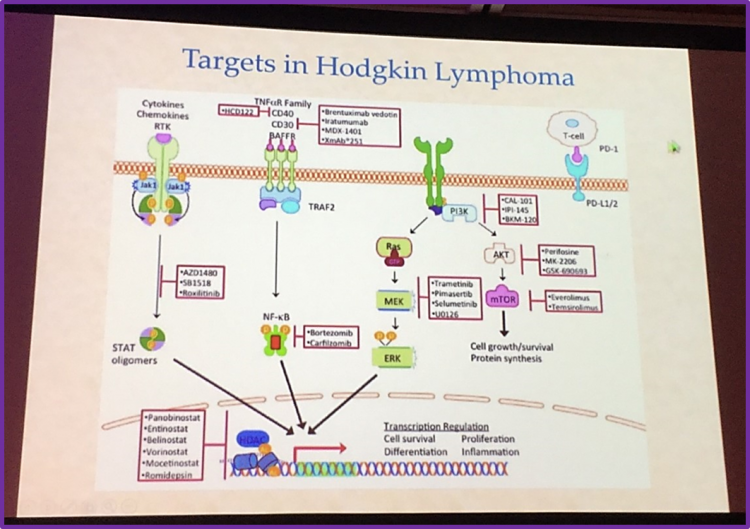
Conversely, in Hodgkin Lymphoma (HL), targeted therapy is proving to be very promising. Brentuximab vedotin, an antibody conjugated to a drug which binds to TNFα receptor CD30, acts by disrupting the cell cycle, ultimately leading to apoptosis of tumor cells. Brentuximab vedotin was granted accelerated approval by the FDA and EMA due to its high activity, safety profile and efficacy in treatment of patients with HL and also with R/R Anaplastic Large Cell Lymphoma (ANCL), a rare entity of NHL. Additionally, in T-cells, activation of PD-1 (Programmed Cell Death Protein 1)/PD-L1 (Programmed Death Ligand 1) can inhibit T-cell cytokine production, effector function, and tumor directed migration thus leading to T-cell tolerance. Anti-PD-1 acts to block the PD-1 signaling pathway, leading to T-cell activation, which can act on tumor cells and lead to apoptosis. Nivolumab, an anti-PD-1 monoclonal antibody, has been shown in clinical trials to have a considerable therapeutic activity in patients with R/R cHL and has been approved by FDA in this setting.
To date, targeted therapy, has shown to be effective in many subtypes of NHL and HL. On-going clinical trial results are encouraging. These emerging strategies for therapy are very promising for therapy in lymphomas.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








