All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ESMO 2016 | Educational Session – MCL: Current and novel therapy
Bookmark this article
Professor of Clinical Hematology, Simon Rule from the Derriford Hospital and Peninsula Medical School, Plymouth, UK, presented a session focused on novel agents for the treatment of Mantle Cell Lymphoma (MCL) at the ESMO congress 2016, at Copenhagen, Denmark.
Professor Simon Rule began his presentation by showing an overview figure on the current first-line therapies for patients with MCL from his paper published with a colleague, Elias Campo, in Blood. The current therapeutic strategies for patients with MCL depends on their fitness and age. For MCL patients that are fit, the treatment option involves a rituximab and high-dose cytarabine based regimen consolidated with an Autologous Stem-Cell Transplant (ASCT). For MCL patients in whom an intensive approach is not feasible, a chemotherapeutic option containing R-CHOP, fludarabine and bendamustine with rituximab maintenance can be used. For less fit MCL patients, a less intensive approach including rituximab alone or CVP, chlorambucil, cladribine or thalidomide, usually in combination with rituximab can be used.
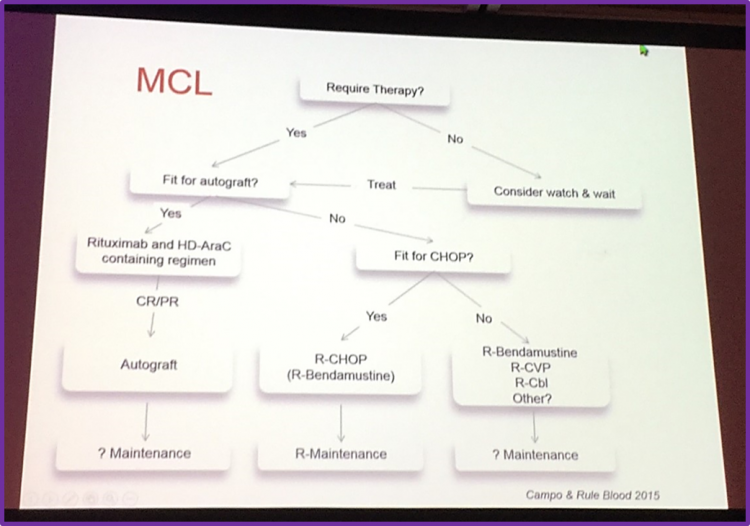
- Intensification in 1st line
Professor Simon Rule then moved on to discuss the NORDIC MCL2 study, which focusses on an intensified first-line therapy for MCL patients. A 2010 update showed an improvement in the Overall and Event Free Survival (OS, EFS).
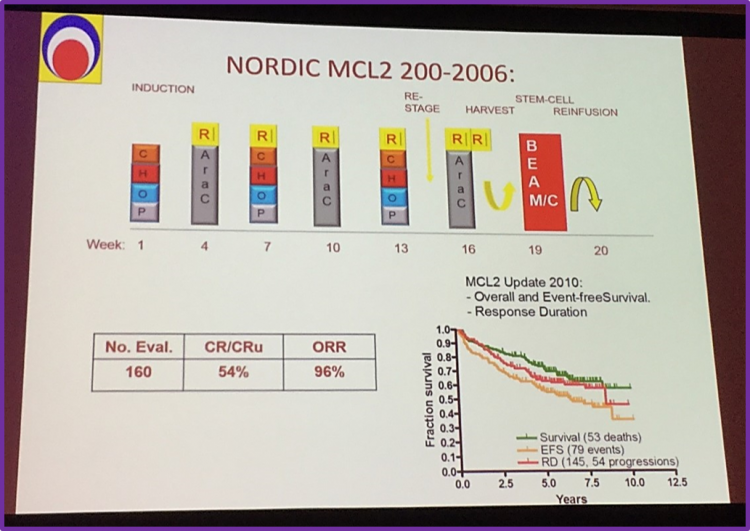
A 15-year follow-up of this study published by Eskelund et al. was discussed. In an ITT based-analysis, the median OS and Progression Free Survival (PFS) were 12.7 and 8.5 years, respectively. The MCL International Prognostic Index (MIPI), including B-miR, significantly divided patients into distinct risk groups.
- Novel agents in MCL
Professor Rule then focused on the use of novel agents in MCL therapy.
- Role of rituximab maintenance
To illustrate this, he outlined the design of the LyMa trial; a randomized prospective trial aiming to assess the benefit of rituximab maintenance after ASCT in young patients with previously untreated MCL (NCT00921414). Patients received 4 doses of R-DHAP followed by ASCT. The conditioning regimen for ASCT was R-BEAM. Patients were then randomly assigned to receive 3 years rituximab maintenance therapy or were put under observation. The results obtained from the study showed that 3 years of rituximab maintenance significantly improved PFS compared to observation.
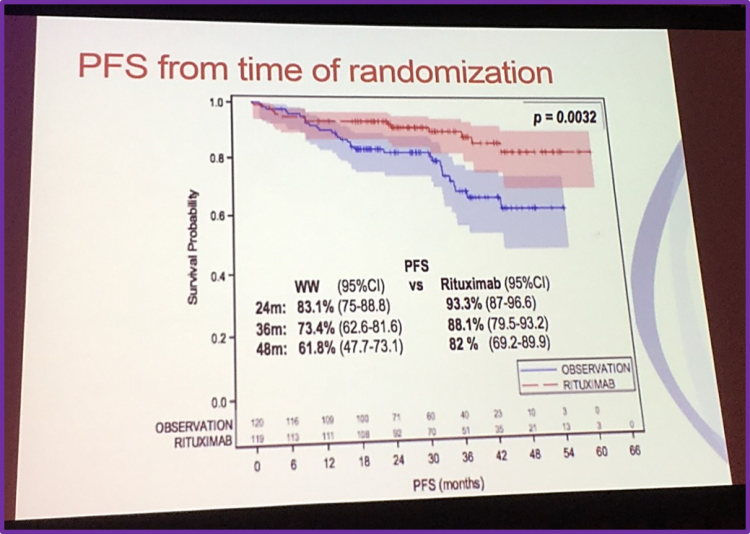
- Role of bortezomib in 1st line
Professor Simon Rule then continued by discussing a phase III trial published by Robak et al. which aimed to assess the use of bortezomib as front-line therapy for patients with MCL. 487 patients randomly received either R-CHOP or VR-CAP. At a median follow-up of 40.4 months, mPFS was 14.4 months in the R-CHOP cohort and 24.7 months in the VR-CAP cohort. Hazard Ratio (HR) favored the VR-CAP group: 0.63 (p< 0.001). This study also found that there was no difference in OS.
| ORR (%) | CR (%) | |
| R-CHOP | 89 | 42 |
| VR-CAP | 92 | 53 |
Robak et al. concluded that VR-CAP was more effective than R-CHOP in patients with newly diagnosed MCL, but at the cost of increased hemo-toxicity.
- Role of lenalidomide in 1st line
He then continued on the use of novel agents for therapy in MCL by discussing a phase II study published by Ruan et al., which evaluated the use of lenalidomide plus rituximab for first-line therapy. At a median follow up of 30 months, in an ITT based-analysis ORR was 87% and CRR 64%. The median 2-year PFS was 85%. Patients with a MIPI score indicating high-risk disease had an unfavorable PFS compared to patients with low-risk disease. The results obtained from this study suggest that a combination therapy consisting of lenalidomide plus rituximab was active as first-line therapy.
| Response | Patients (no.) | IIT Population (N = 38) (%) | Patients Who Could Be Evaluated (N = 36) (%) |
| OR | 33 | 87 | 92 |
| CR | 23 | 61 | 64 |
| PR | 10 | 26 | 28 |
| SD | 1 | 3 | 3 |
| PD | 2 | 5 | 6 |
| Could not be evaluated | 2 | 5 | - |
- Role of ibrutinib
Professor Simon Rule then focused on Bruton’s Tyrosine Kinase (BTK), a critical tyrosine kinase for lymphoma cell survival and proliferation, and its inhibitors such as ibrutinib.
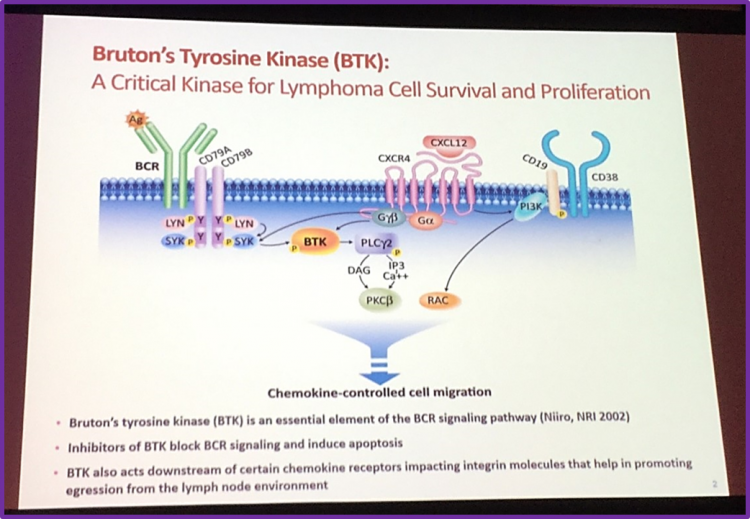
It was exmplained that ibrutinib (PCI-32765) is a highly potent BTK inhibitor (inhibition at IC50 = 0.5nM); it forms a bond with cysteine-481 in BTK. Ibrutinib has a high degree of specificity for hematopoietic cells and provides durable BTK inhibition, despite rapid drug elimination.
Firstly, to illustrate the efficacy of ibrutinib, Rule discussed the PCYC-1104-CA phase II trial (NCT01236391), in R/R MCL patients. At 6.2 months, the OR and CR rates were 64% and 50.5%, respectively.
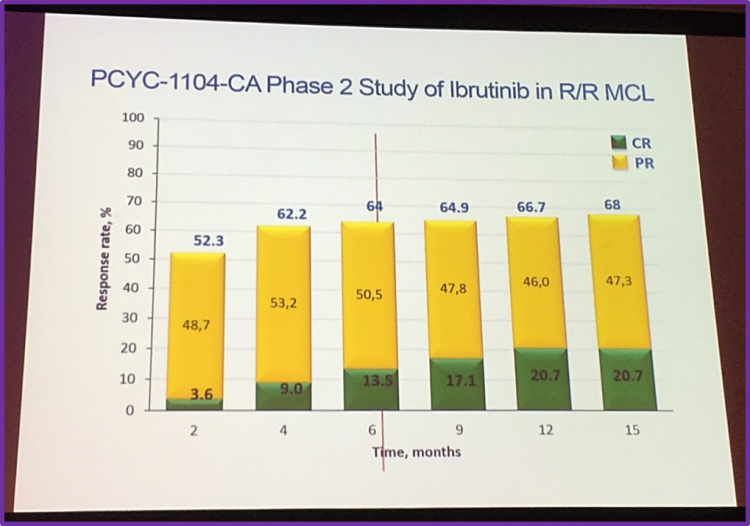
Then, he presented a pooled analysis of 3 studies (PCYC-1104, MCL2001 and MCL3001) using ibrutinib in R/R MCL patients with an aim to assess the impact of the baseline factors on OS. 370 patients with a median age of 68 were included in the analysis. 94% of these patients had an ECOG 0–1, 45% and 32% had intermediate and high-risk sMIPI (simplified MCL International Prognostic Index), respectively. Most patients had 1–3 lines of prior therapy and non-blastoid histology.
| PCYC-1104 (N = 111) | SPARK (N = 120) | RAY (N = 139) | Pooled (N = 370) | |
| Median age, years | 68 | 67.5 | 67 | 67.5 |
|
ECOG performance status, % 0-1 2 >2 |
89 10 1
|
91 9 0 |
99 1 0 |
94 6 1 |
| Blastoid histology, % | 15 | 9 | 12 | 12 |
| Bulky disease (≥5cm), % | 39 | 53 | 54 | 49 |
|
sMIPI, % Low risk (1-3) Intermediate risk (4-5) High risk (6-11) |
14 38 49 |
24 48 28 |
32 47 22 |
24 45 32 |
|
Median prior lines of treatment (range) |
3 (1-5) | 2 (1-8) | 2 (1-9) | 2 (1-9) |
At a median follow up across the 3 studies of 24.25 months, the median PFS and median OS were 12.8 months and 25 months, respectively. The 2-year PFS and OS of patients who had received 1 prior line of therapy were 57% and 68%, respectively; (2 lines of therapy: 2-y PFS = 29% and 2-y OS = 50%; 3 lines of prior therapy: 2-y PFS = 18% and 2-y OS =31%). The data suggests that patients who had received 1 prior line of therapy had the longest PFS and OS. For patients who achieved a CR, mPFS and mOS had not been reached. 92% of patients who achieved CR were alive at 2 years. In patients that achieved a PR, mPFS and mOS were 20.1 months and 25 months, respectively. In patients with blastoid histology, mPFS and mOS were shorter compared to non-blastoid histology patients: 5.1 vs 14.6 months and 12.75 months vs not reached, respectively.
- Combination therapy with ibrutinib
Professor Simon Rule then focused his talk at the evolving combination with ibrutinib. Firstly, he discussed a phase II trial by Wang et al., which involved the combination of ibrutinib and rituximab in patients with relapsed MCL. The preliminary data indicates that ibrutinib plus rituximab is well tolerated and effective in patients with Ki-67 less than 50%.
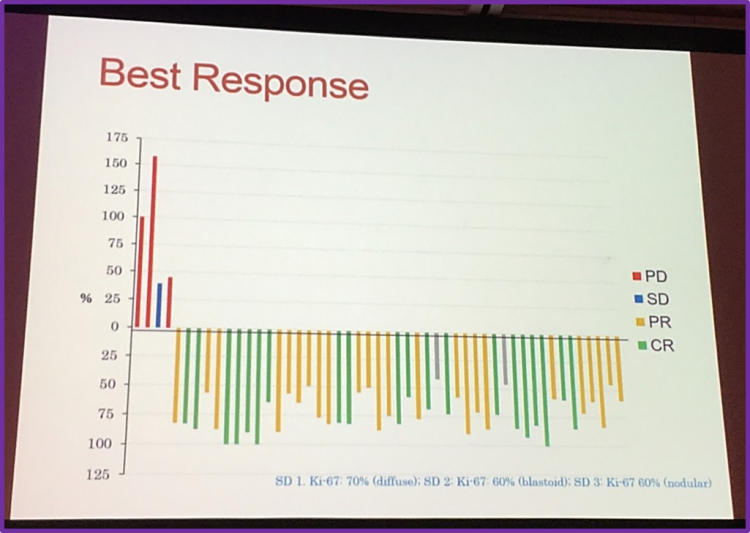
Moreover, he discussed a phase1/1b study published by Maddocks et al. that aimed to evaluate the efficacy of ibrutinib combined with rituximab and bendamustine in patients with previously untreated and R/R Non-Hodgkin Lymphoma (NHL). The CRR and ORR in patients with MCL were 76% and 94%, respectively.
| Histology | No. evaluable patients | CR (%) | PR (%) | OR (%) |
| MCL | 17 | 13 (76) | 3 (18) | 16 (94) |
| DLBCL | 16 | 5 (31) | 1 (6) | 6 (37) |
| FL | 10 | 5 (50) | 4 (40) | 9 (90) |
| MZL | 1 | 0 | 1 (100) | 1 (100) |
| Transformed Lymphoma | 2 | 1 (50) | 0 | 1 (50) |
| All Patients | 46 | 24 (52) | 9 (20) | 33 (72 |
Professor Simon Rule continued his talk by discussing current clinical trials in 1st line with ibrutinib, including MCL 3002-SHINE (NCT01776840); a phase III study aimed at evaluating the efficacy and safety of ibrutinib in combination with rituximab and bendamustine in patients 65 years or older with newly diagnosed MCL.
He discussed another ongoing prospective trial, ENRICH, a randomized open label phase III study comparing two treatments in previously untreated elderly patients with MCL: ibrutinib plus rituximab, versus rituximab and chemotherapy.
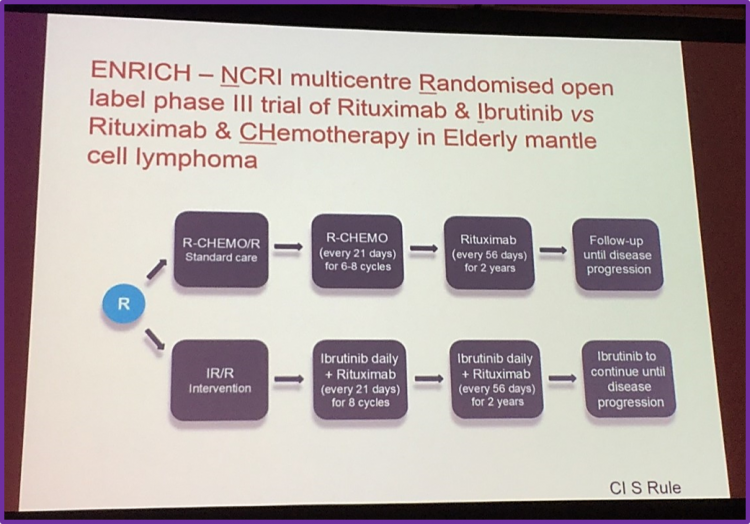
Professor Simon Rule mentioned new generation BTK inhibitors, including ONO 4059, ACP 196, and M 7583.
- Role of venetoclax
He then moved on to discuss another novel agent in MCL therapy, venetoclax. He presented a phase 1 study by Gerecitano et al. focused on the use of venetoclax monotherapy in patients with R/R NHL. In MCL patients, the median PFS was 14 months.
| Median PFS, Months (95% CI) | |
| All, n = 106 | 17 (14.22) |
| MCL, n = 28 | 14 (ND) |
| FL, n = 29 | 11 (6.19) |
| DLBCL, n = 34 | 1 (1.3) |
Professor Simon Rule concluded his talk at the ESMO Congress by suggesting that novel agents will have a major impact on therapy for MCL patients and the use of combination therapy in MCL patients should soon become part of the standard therapy. He also added that early use of therapy can impact the survival of MCL patients.


Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








