All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
ESMO 2016 | Selected Oral Presentations: EMZL – clarithromycin dose for treating EMZL is 1g/day
Bookmark this article
Dr Caterina Cecchetti, from the San Raffaele Scientific Institute in Milan, Italy, and colleagues presented during the ESMO congress in October 2016 in Copenhagen, Denmark, a retrospective study on 55 patients with Extranodal Marginal Zone Lymphoma (EMZL) treated by clarithromycin monotherapy.

The study had three treatment schedules: 500mg clarithromycin twice per day for six months (n=13), three courses of 500mg twice per day for 21 days, every 35 days (n=19), and four courses of 2g per day for 14 days, every 21 days (n=23). The primary outcome was the efficacy and safety of the treatment schedules.
The authors presented data showing that the Overall Response Rate (ORR) of the 1g/day and 2g/day treatment groups were 40% and 46% respectively (P=0.28). Data presented also showed that the Complete Response (CR) rate of the 1g/day and 2g/day treatment groups were 12% and 39%, respectively (P=0.02).
| Response | N(%) | 1 g/day - 32 pts (%) | 2 g/day - 23 pts (%) | P |
| CR | 13 (24) | 4 (12) | 9 (39) | 0.02 |
| PR | 13 (24) | 9 (28) | 4 (17) | |
| ORR | 26 (47) | 13 (40) | 13 (56) | 0.28 |
| SD | 16 (29) | 12 (37) | 4 (17) | |
| PD | 13 (23) | 7 (21) | 6 (26) |
The reported overall Progression Free Survival (PFS) rate, with median follow-up time of 33 months, was found to be 52 ± 7%. The PFS for each treatment group was 60 ± 9% (1g/day) and 42 ± 10% (2g/day).
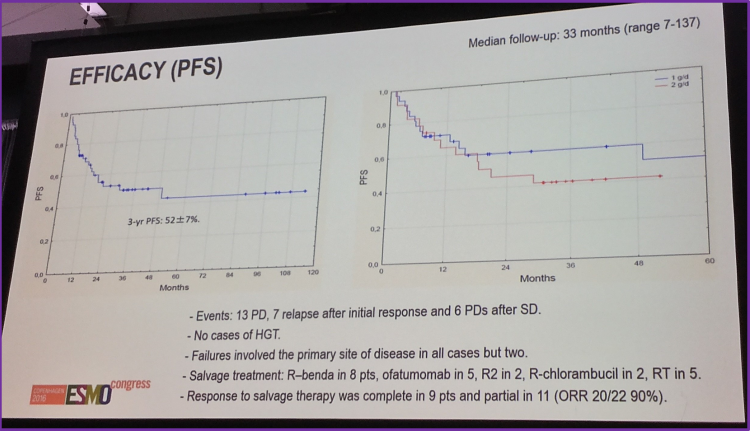
There was one significant difference in the toxicity profile: the rate of nausea in the 1g/day and 2g/day groups which had a rate of 25% and 52%, respectively (P=0.03). There were no incidence of any grade 4 side effects in either dosage group.
| Clarithro 1 g/day - 32 patients (%) | |||||
| Side effects | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade |
| Nausea | 5 | 2 | 1 | 0 | 8 (25) |
| Disgeusia | 4 | 1 | 0 | 0 | 5 |
| Dizziness | 2 | 2 | 0 | 0 | 4 |
| Headache | 1 | 0 | 0 | 0 | 1 |
| Arthralgia | 0 | 1 | 0 | 0 | 1 |
| Rash | 0 | 1 | 0 | 0 | 1 |
| Clarithro 2 g/day - 23 patients (%) | |||||
| Side effects | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade |
| Nausea | 7 | 3 | 2 | 0 | 12 (52) |
| Disgeusia | 2 | 0 | 0 | 0 | 2 |
| Dizziness | 4 | 0 | 0 | 0 | 4 |
| Headache | 2 | 0 | 0 | 0 | 2 |
| Arthralgia | 0 | 1 | 0 | 0 | 1 |
| Rash | 1 | 0 | 0 | 0 | 1 |
The main conclusion from this presentation was that in future the recommended dose should be 1g/day to balance efficacy and tolerability. At the end of the presentation a new trial of clarithromycin was discussed. The CLEO trial is a phase II trial using 1g/day clarithromycin in combination with lenalidomide in patients with R/R EMZL.

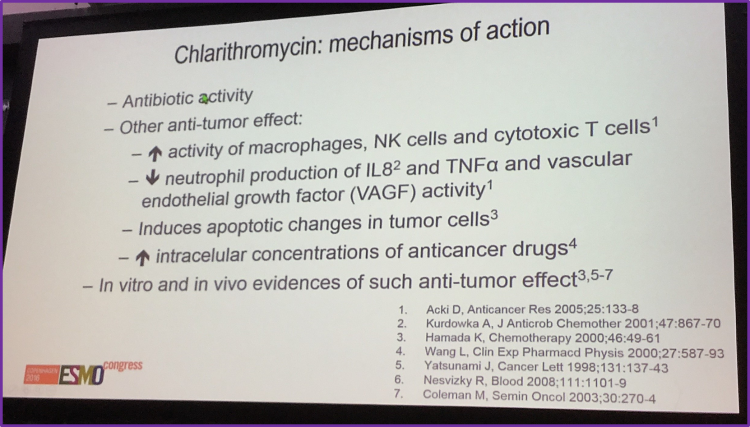
Following Dr Caterina Cecchetti’s presentation, a discussion session was held, led by Dr Armando López-Guillermo from Instituto Oncológico Baselga, Barcelona, Spain. Dr López-Guillermo commented on the anti-tumor mechanisms of action of clarithromycin which included increasing the activity of macrophages and reducing neutrophil production of Vascular Endothelial Growth Factor (VEGF).
More about...

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








