All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
iwCLL 2017 | Final results of the phase II ICLL03 RICAC-PMM trial of RIC allo-SCT for high-risk patients
Bookmark this article
On 15th May 2017, during iwCLL, the second half of the “Additional Therapies for the Relapsed/Refractory CLL Patient” session was jointly chaired by Guillermo Dighiero (Unité d'Immunohématologie et d'Immmunopathologie, Institut Pasteur) and Federico Caligaris-Cappio (Università Vita-Salute San Raffaele).
Olivier Tournilhac, MD, PhD, from CHU Estaing, Clermont-Ferrand, France, gave a talk titled “RIC allogeneic stem cell transplantation for high risk CLL followed by pre-emptive MRD-based immune intervention. Phase II ICLL03 RICAC-PMM trial: final results” during this session.
The ICLL003 RICAC-PMM study (NCT01849939) is a multicenter, phase II study by the FILO and SFGM-TC groups, and aimed to increase the rate of MRD-negativity at 12 months post-ASCT using a pre-emptive, MRD based, immune intervention algorithm. MRD-negativity was assessed by 10 color flow cytometry, and was defined as <0.01% (10-4) in blood and bone marrow. Between September 2012 and February 2015, 43 patients were included from 16 centers in France; 42 patients were included in the analysis (1 patient had no ASCT, due to donor comorbidities).
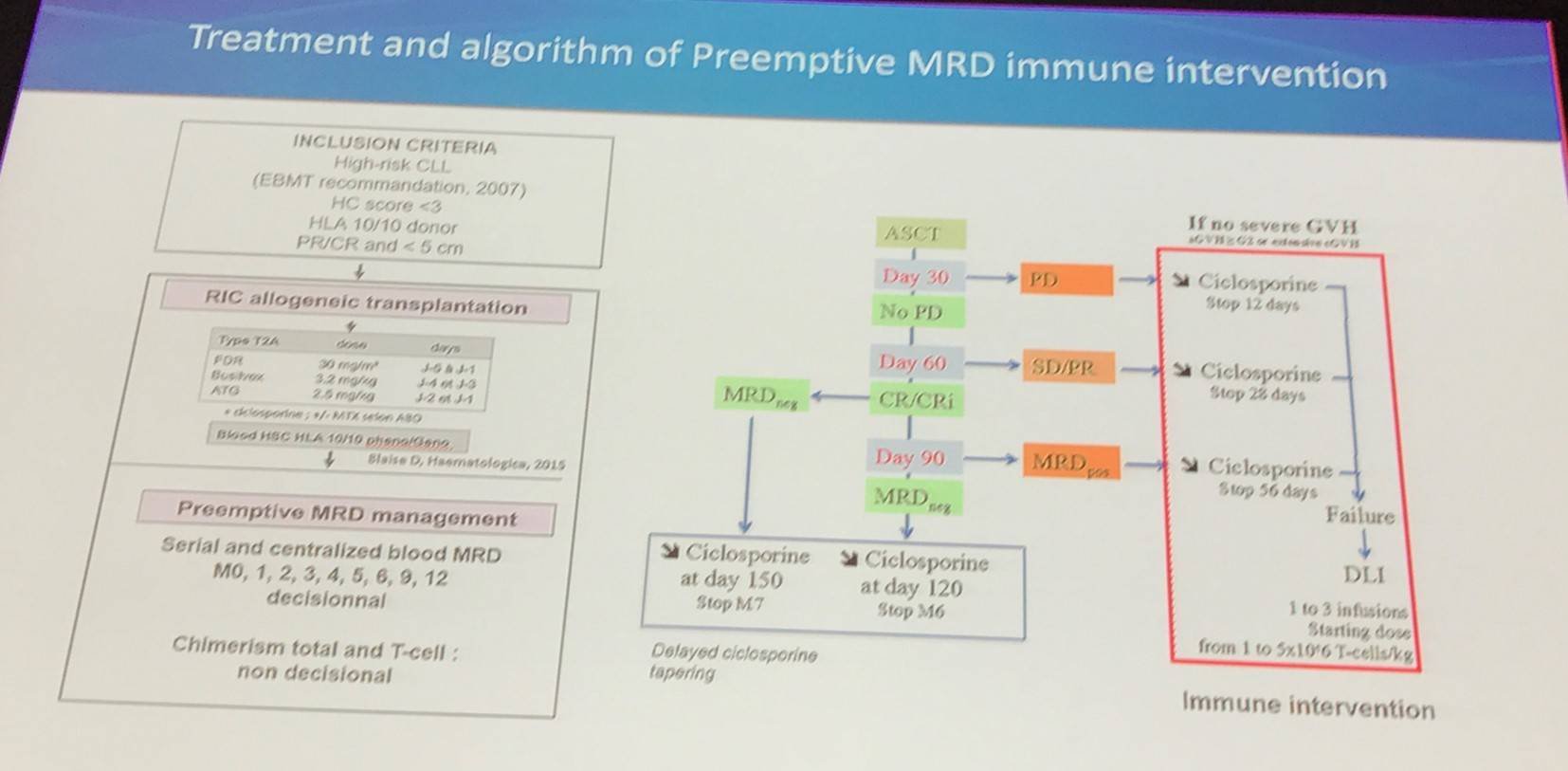
At transplantation:
- Median age = 58 years (range, 40–68)
- Time from diagnosis to transplant = 4.4 years (range, 0.2–14.7)
- Median number of prior therapies = 2 (range, 1–5) including at last line: alemtuzumab (n=17), immunochemotherapy (n=21), and BCR inhibitor (n=4)
- Response: PR = 78%; CR/CRi = 22%
- Pre-ASCT MRD = 0.78% (0.005–70) and undetectable in 6 patients
- Allogeneic transplantation per the EBMT 2007 criteria
|
*including 1 patient with complex karyotype but 17p deletion, eventually ruled out (TP53 wild type) |
|
|
Del(17p) first-line* |
11 (26%) |
|
Del(17p) R/R |
16 (38%) |
|
Relapse <2 years, following fludarabine combination and no del(17p) |
15 (36%) |
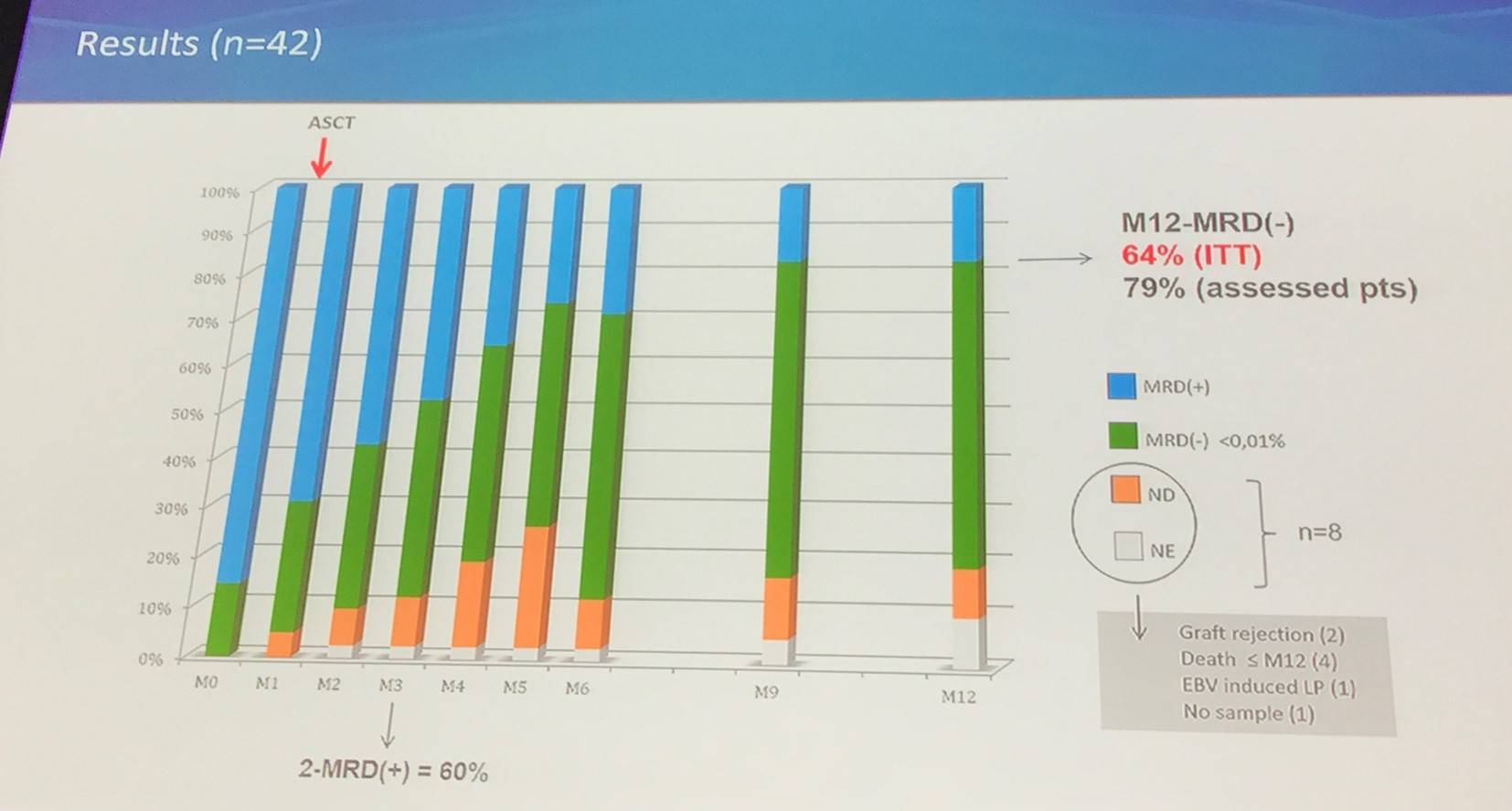
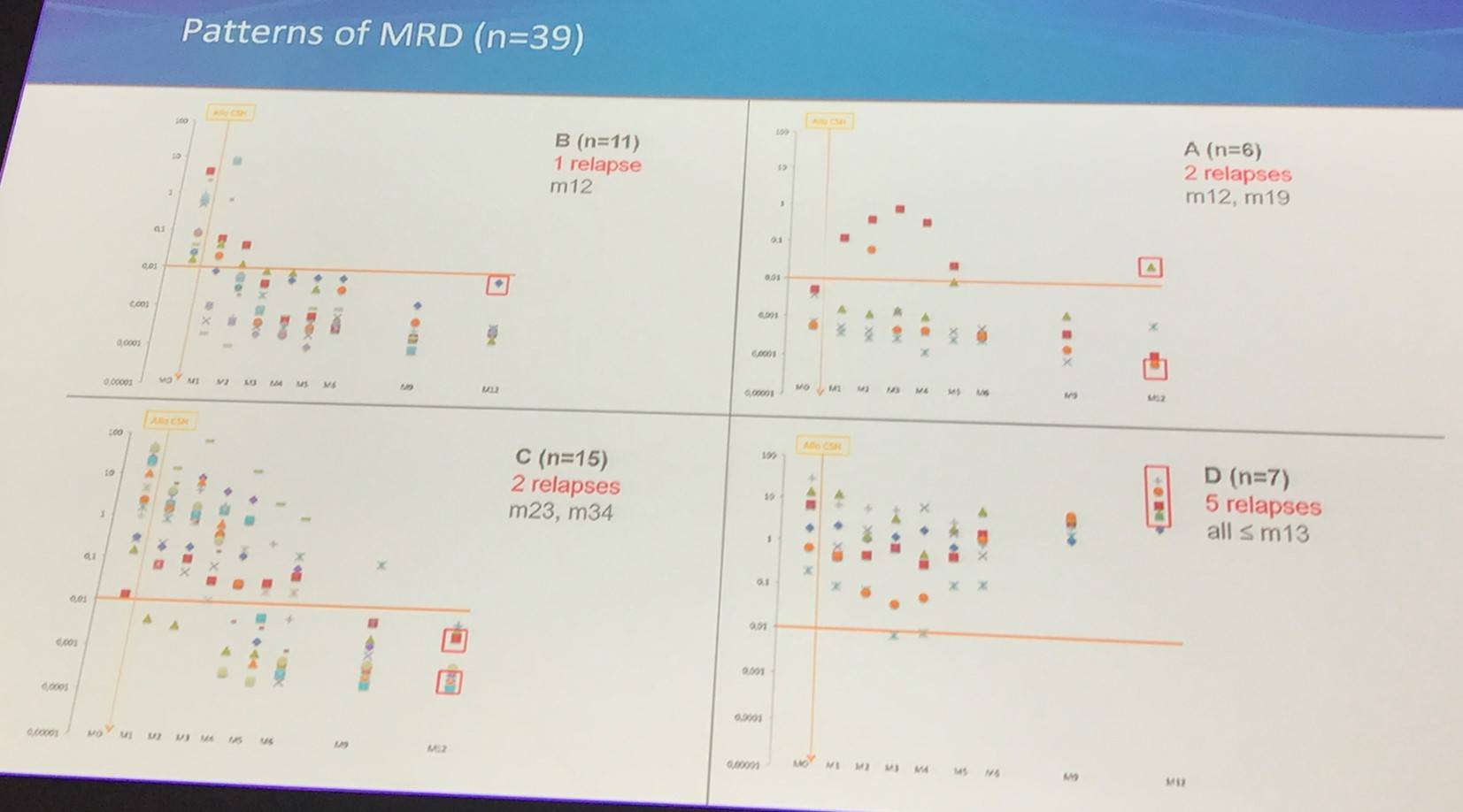
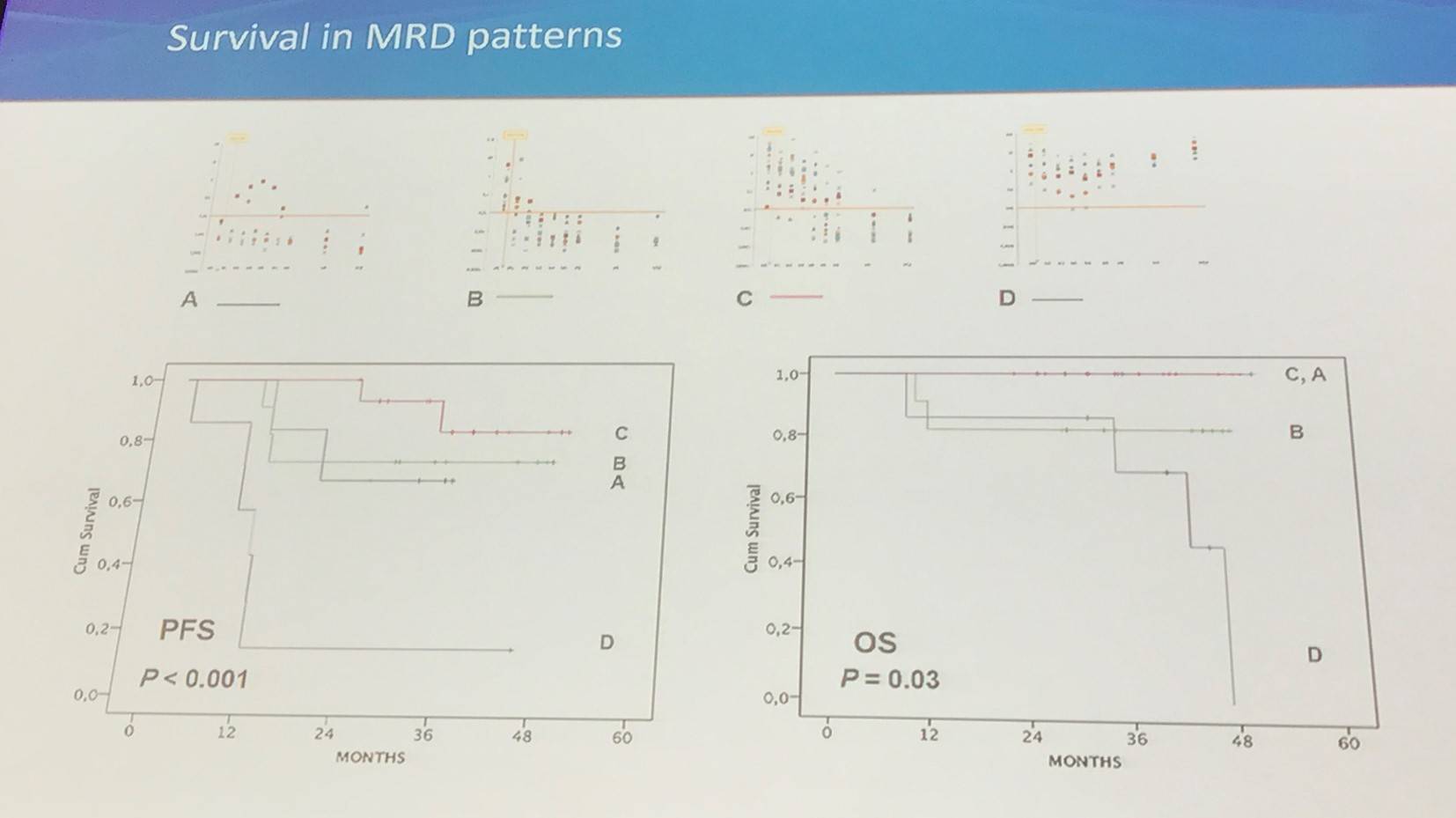
A: M0 MRD(-); B: M0 MRD (+), no need of immune intervention; C: M0 MRD(=), immune intervention - CSA withdrawal; D: M0 MRD(+), immune intervention – CSA withdrawal and DLI
At 24 months:
- Follow-up = 36 months (19–53)
- PFS = 66.7% (95% CI, 51.5–79.0%)
- OS = 90.5% (95% CI, 77.9–96.2%)
- NRM = 7.1% (95% CI, 3.1–11.1%)
- 7 deaths reported, 4 due to toxicity and 3 due to Richter syndrome
In terms of safety:
- aGvHD ≥Grade 2 in 26% of patients; Grade 3 in 10%
- Limited cGvHD in 38% of patients (95% CI, 23–53); Extensive cGvHD in 23% of patients (95% CI, 10–36)
- Including cGvHD in 2 patients following immune intervention (n=1 DLI, n=1 CSA withdrawal)
- 4 toxic deaths reported (CMV/EBV in month 1, GvHD in months 6 and 10, and pulmonary aspergillosis/pneumocystosis infection in month 9)
Olivier Tournilhac concluded his talk with a concise summary slide:

- Tournilhac O. RIC allogeneic stem cell transplantation for high risk CLL followed by pre-emptive MRD-based immune intervention. Phase II ICLL03 RICAC-PMM trial: final results. XVII International Workshop on Chronic Lymphocytic Leukemia; 2017 May 12–15; New York, USA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








