All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
EHA 2017 | First-line treatment for aggressive NHL: Mantle Cell and Diffuse Large B-Cell Lymphoma
Bookmark this article
On Friday 23rd June, during the 22nd Congress of the European Hematology Association (EHA), a session took place discussing first-line therapy for aggressive Non-Hodgkin Lymphoma (NHL). The session was co-chaired by Adolfo la Fuente Burguera from the MD Anderson Cancer Center, Madrid, Spain, and Martin Dreyling from the University Hospital of Munich, Germany.
Abstract S105
The first abstract was presented by Steven Le Gouill, MD, PhD, from Nantes Medical University, Nantes, France, and conveyed final results from the randomized, open-label, phase III LyMa trial (NCT00921414).
Currently, there is no cure for patients with Mantle Cell Lymphoma (MCL). Despite high Complete Response (CR) rates after frontline immunochemotherapy induction and subsequent Autologous Stem Cell Transplantation (ASCT), MCL patients experience numerous relapses.
The aim of the LyMa trial was to evaluate whether or not rituximab maintenance (375mg/m2 every 2 months for 3 years) after ASCT prolonged response duration. The primary outcome measure was 4-year Event Free Survival (EFS) after rituximab maintenance. Secondary endpoints included Progression-Free Survival (PFS) and Overall Survival (OS) of the whole group, as well as Complete Response (CR), Partial Response (PR), and Overall Response Rate (ORR) after induction with R-DHAP and after ASCT. Overall, 299 patients, younger than 66 years (median, 57; range, 27–65) at diagnosis, were included; of whom 240 were randomly assigned to rituximab maintenance or observation after ASCT (120 patients each).
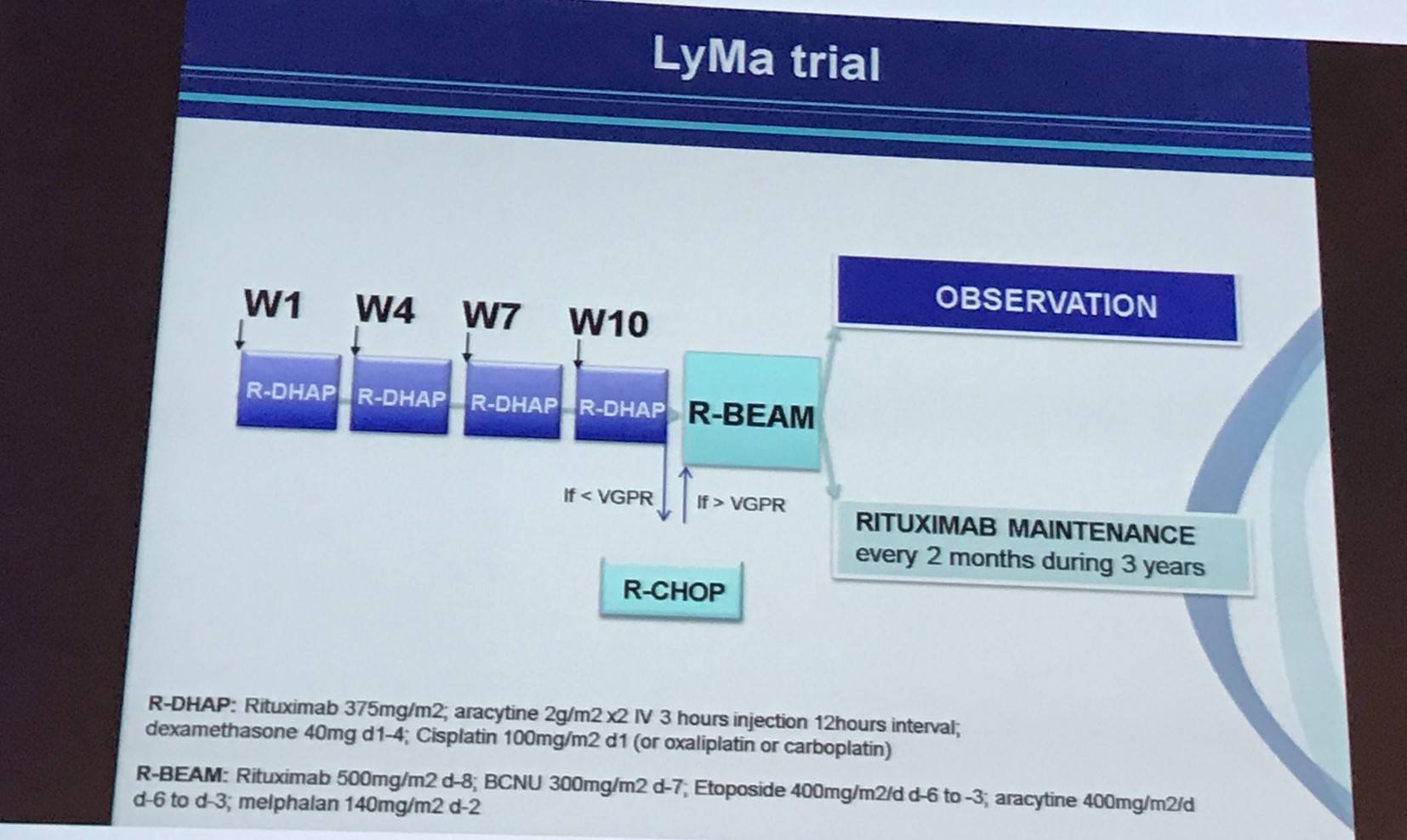
Le Gouill summarized the inclusion and exclusion criteria:
|
Inclusion criteria |
Exclusion criteria |
|---|---|
|
· Diagnosis of MCL according to the WHO 2009 classification |
· Other types of Lymphoma besides MCL |
|
· Presence of the t(11;14) translocation demonstrated by karyotyping, FISH, molecular biology, or immunochemistry (bcl-1+ / CCND1+) |
· Patients with relapse
|
|
· Untreated MCL patients with at least one tumor site for assessment (not isolated BM or spleen involvement) |
· HIV+ or active hepatitis C or B |
|
· Aged between 18 to 65 years inclusive |
· Poor PS ≥3 |
|
· Signed informed consent |
· Contraindication to the use of the drugs contained in the regimen |
|
|
· Uncontrolled diabetes |
At inclusion, 6%, 10.5%, and 83.5% had Ann Arbor stage II, III, and IV, respectively. The proportion of patients with an ECOG PS of <2 was 94.3% and 29.8% had B symptoms. Bone marrow involvement and LDH elevation was observed in 64.5% and 38.5%, respectively. The proportion of patients with low-, intermediate- and high-risk MIPI score was 53%, 27.5%, and 19.5%, respectively.
Le Gouill outlined the disease status of patients after R-DHAP and after ASCT:
|
Disease status (according to Cheson 99) n, % |
Observation n=120 |
Rituximab maintenance n=120 |
|---|---|---|
|
After R-DHAP |
||
|
ORR |
117 (97.5) |
119 (99.2) |
|
CR/CRu |
104 (86.7) |
102 (85.0) |
|
After ASCT |
||
|
ORR |
120 (100) |
119 (99.2) |
|
CR/CRu |
110 (91.7) |
113 (94.2) |
In the Observation arm, 37 progressions, 25 deaths, and 4 sever infections were reported. In the rituximab maintenance arm, 83 patients completed maintenance and there were 16 progressions, 14 deaths, and 4 severe infections were reported.
Median follow-up from randomization after ASCT was 50.2 months (range, 46.4–54.2). Starting from randomization, 4-year EFS was 78.9% (95% CI, 69.5–85.6) for rituximab maintenance compared to 61.4% (95% CI, 51.3–69.9) for observation (P = 0.0012). 4-year PFS was 82.2% (95% CI, 73.2–88.4) for rituximab maintenance versus 64.6% (95% CI, 54.6–73.0) for observation (P = 0.0005) and OS was 88.7% (95% CI, 80.7–93.5) for rituximab maintenance compared to 81.4% (95% CI, 72.3–87.7) for observation (P = 0.0413). The death rate for patients who received rituximab maintenance was lower and they were less likely to die (HR, 0.5; 95% CI, 0.255–0.986) than patients in the observation arm.
Le Gouill concluded the talk by stating that the LyMa trial showed for the first time that maintenance therapy with rituximab after ASCT prolongs EFS, PFS, and OS. Therefore, 4 courses of R-DHAP plus ASCT (without TBI) followed by one infusion of rituximab maintenance every 2 months for 3 years is a new standard of care for young patients with MCL.
Abstract S106
Hervé Tilly, MD, PhD, from Centre Henri Becquerel, University of Rouen, Rouen, France, gave the second talk which discussed polatuzumab vedotin (pola) plus rituximab (R), cyclophosphamide, doxorubicin, prednisone (CHP) for patients with newly diagnosed Diffuse Large B-Cell Lymphoma (DLBCL).
Pola is an Antibody Drug Conjugate (ADC) containing the potent microtubule inhibitor MMAE conjugated to a CD79b monoclonal antibody via a protease-cleavable peptide linker. Pola as monotherapy and in combination with anti-CD20 antibodies such as rituximab has shown promising efficacy in R/R DLBCL.
|
Treatment regimen |
Best Overall Response |
Reference |
|---|---|---|
|
Pola 1.8–2.4mg/kg |
51% |
|
|
Pola 1.8–2.4mg/kg + rituximab |
56% |
Tilly outline the GO29044 study design (NCT01992653), a phase Ib/II trial aiming to determining the safety, tolerability, and anti-tumor activity of pola-R-CHP. Patients were included if they were aged 18 years or older, had a measurable lesion, and had an ECOG PS of 0–2. The initial dose-escalation portion showed an acceptable safety profile and established a recommended phase II dose of pola at 1.8mg/kg. During this talk, updated safety and efficacy results for the phase II dose in 45 previously untreated DLBCL patients was presented.
|
Drug |
Route |
Dose |
Days |
|---|---|---|---|
|
Rituximab |
IV |
375mg/m2 |
1 |
|
Cyclophosphamide |
IV |
750mg/m2 |
1 |
|
Doxorubicin |
IV |
50mg/m2 |
1 |
|
Prednisone |
PO |
100mg/day |
1–5 |
|
Polatuzumab vedotin |
IV |
1.8mg/kg |
2 (cycle 1 and cycle 2) 1 (subsequent cycles) |
Six to eight cycles of Pola-R-CHP at 21-day intervals were planes, and response was evaluated by CT and PET after the fourth cycle and at the end of treatment. At baseline, the median age of the 45 patients included was 69 years (range, 45–80) and 67% and 33% had an ECOG PS score of 0–1 and 2, respectively. Thirty-seven patients (82%) had stage III/IV disease and 38% had an IPI score of 4–5. Cell of Origin (COO) was available for 34 patients: 12 (35%) were Activated B-Cell-like (ABC), 17 (50%) were Germinal Center B-Cell-like (GCB), and 5 patients (15%) were unclassified. All 45 patients received one or more doses of study drug.
In total, 40 patients completed 6 (23 patients) or 8 (17 patients) cycles; all patients experienced at least one Adverse Event (AE). Grade 3–4 AEs occurred in 58%, and one patient experienced a grade 5 atrial fibrillation with cardiac failure. Grade 3–4 neutropenia and febrile neutropenia were reported in 27% (12 patients) and 11% (5 patients). Grade 3–4 Infections and infestations were seen in 11% (5 patients) and thrombocytopenia was experience by 2 patients (4%). Serious Adverse Events (SAEs) were observed in 17 patients (38%) including 3 events of febrile neutropenia, 5 infections and infestations, and 2 pulmonary embolisms. Peripheral neuropathy occurred in 18 (40%) patients. Among these patients, 12 (27%) were grade 1, 4 (9%) were grade 2, and 2 (4%) were grade 3. All grade 2–3 peripheral neuropathy attributed to pola occurred at cycle 5 or later. AEs resulting in a reduced dose of pola was reported in 6 patients (13%), and AEs causing discontinuation of chemotherapy occurred in 3 patients (7%).
Tilly then presented data on PET response at the end of treatment:
|
|
Overall (n=45) |
By IPI; n,% |
COO available (n=34); n,% |
||||
|---|---|---|---|---|---|---|---|
|
|
Pola-R-CHP; n,% |
90% CI |
IPI 0–2 (n=10) |
IPI 3–5 (n=35) |
ABC (n=12) |
GCB (n=17) |
U (n=5) |
|
ORR |
41 (91) |
(81–97) |
10 (100) |
31 (89) |
12 (100) |
17 (100) |
3 (60) |
|
CR |
35 (78) |
(65–87) |
10 (100) |
25 (71) |
11 (92) |
15 (88) |
2 (40) |
|
PR |
6 (13) |
(6–25) |
- |
6 (17) |
1 (8) |
2 (12) |
1 (20) |
|
PD |
3 (7) |
(2–16) |
- |
3 (9) |
- |
- |
2 (40) |
|
Unevaluable/missing |
1 (2) |
(0–10) |
- |
1 (3) |
- |
- |
- |
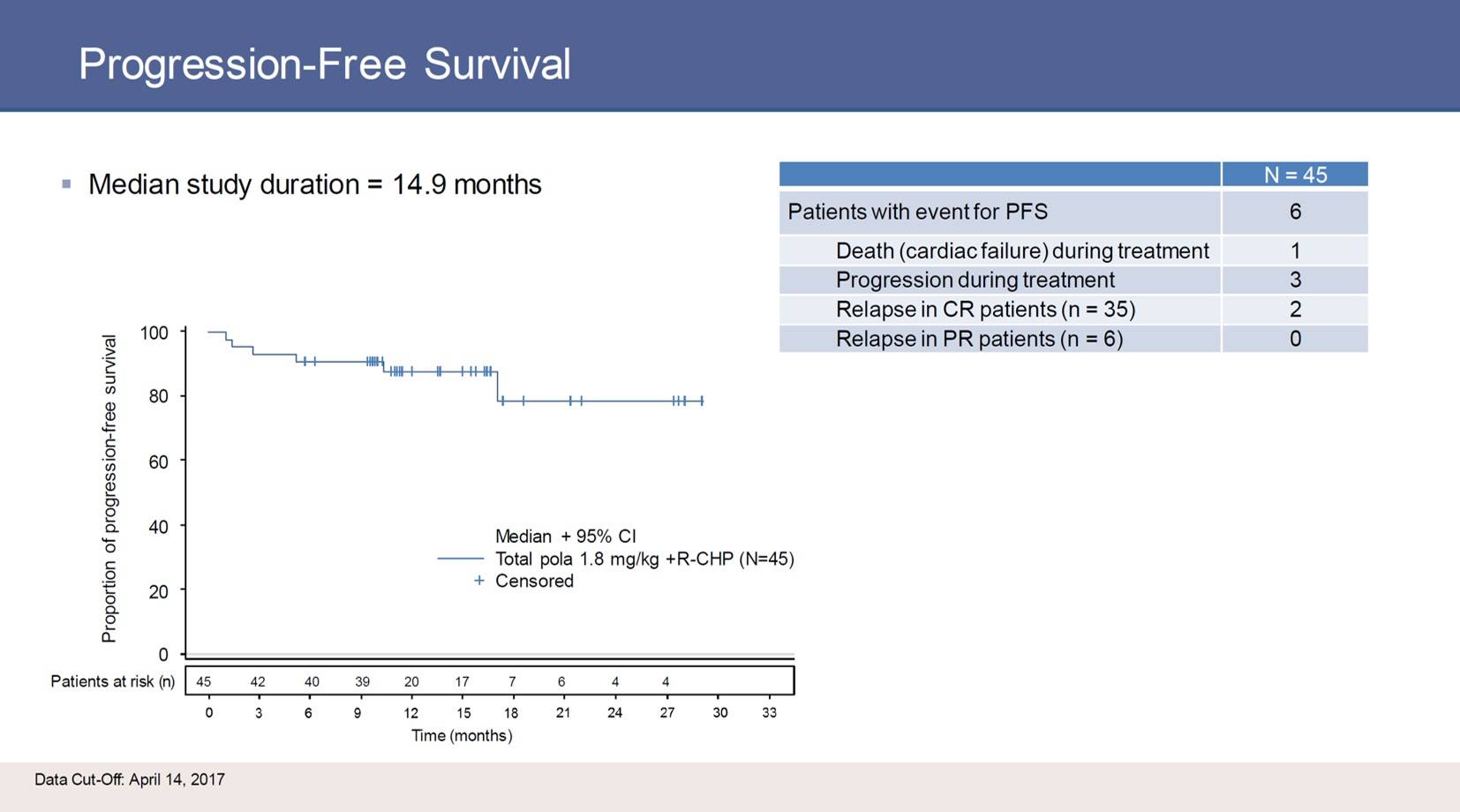
Tilly concluded his talk by stating that pola at 1.8mg/kg plus R-CHP as upfront therapy for newly diagnosed DLBCL has an acceptable safety profile and at the end of treatment demonstrated some encouraging response rates. Most patients represented a poor prognosis group by age and IPI. The treatment response to this regimen should perhaps be further investigated.
Abstract S107
The third that the LH attended in this session was presented by Pieternella Lugtenburg, MD, PhD, from the Erasmus MC Cancer Institute, Rotterdam, The Netherlands, and discussed Subcutaneous (SC) or Intravenous (IV) rituximab in combination with CHOP for patients with newly diagnosed DLBCL.
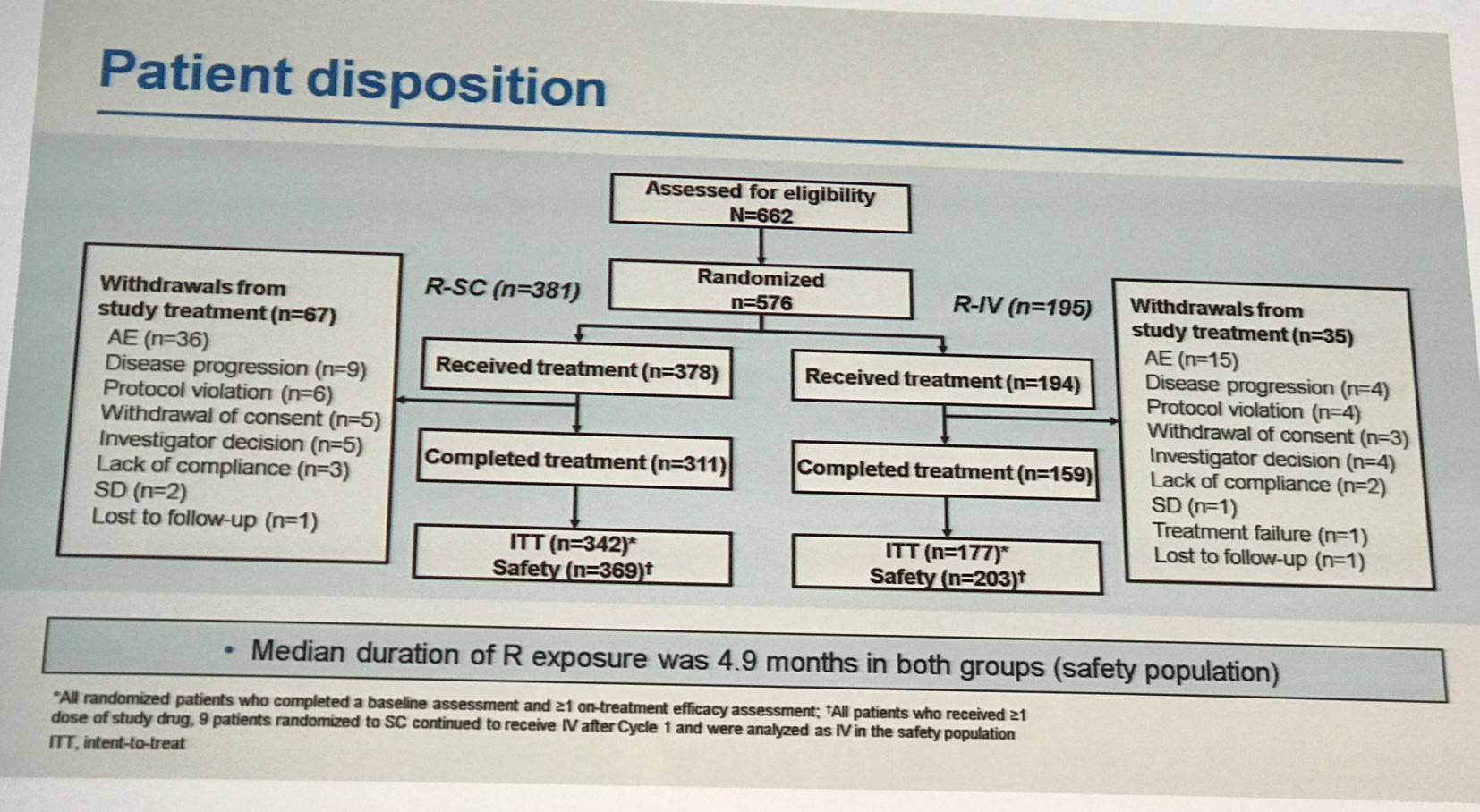
Rituximab administered by IV plus CHOP is the standard first-line therapy for DLBCL. It is thought that the SC formulation (same active ingredient, 12-fold concentrated, co-formulated with recombinant human hyaluronidase) could simplify treatment, reduce burden, and improve patient satisfaction. The phase III SABRINA trial (NCT01200758), reported on by the LH in May of this year, found that both IV and SC administration methods deliver the same amount of rituximab and result in similar ORRs, CRs, and toxicity profiles. Lugtenburg presented final results from the phase III MabEase study investigating the efficacy and safety of CHOP combined with either SC or IV rituximab in patients newly diagnosed, CD20+, DLBCL patients aged 18–80 years (NCT01649856).
The primary outcome measure of the trial was investigator assessed CR/CRu and secondary endpoints include PFS, EFS, Disease Free Survival (DFS), OS, safety, patient treatment satisfaction (RASQ), and time savings (administration time and chair/bed/hospital time). Patients were randomized 2:1 to rituximab SC (R-SC; IV 375mg/m2 cycle 1; SC 1,400mg cycles 2–8) or IV (R-IV; 375mg/m2 cycles 1–8) plus CHOP every 14 or 21 days. Baseline characteristics were well balanced between treatment arms.
Lugtenburg summarized the investigator-assessed responses at end of induction:
|
Efficacy endpoint |
R-SC plus CHOP (n=342); % (95% CI) |
R-IV plus CHOP (n=177); % (95% CI) |
|---|---|---|
|
CR/CRu |
50.6 (45.3–55.9) |
42.4 (35.1–49.7) |
|
PR |
31.6 (26.7–36.8) |
35.6 (28.6–43.1) |
|
PD |
3.8 (2.0–6.4) |
6.2 (3.1–10.8) |
|
ORR |
82.2 (77.7–86.1) |
78.0 (71.1–83.8) |
Median follow-up was 35 months. At database lock, 72.2% of R-SC and 78.5% of R-IV patients had not experienced progression, relapse, or death. Median PFS, EFS, and OS were not reached in either arm and no statistically significant differences were observed between treatment arms. Time-to-event endpoints (PFS, EFS, and OS) at 2-years were then presented:
|
Survival; % (95% CI) |
R-SC plus CHOP (n=342) |
R-IV plus CHOP (n=177) |
|---|---|---|
|
PFS |
75.0 (69.9–79.4) |
81.5 (74.7–86.6) |
|
EFS |
68.6 (63.3–73.4) |
73.4 (66.0–79.4) |
|
OS |
87.4 (83.2–90.5) |
88.0 (82.0–92.1) |
Treatment-emergent AEs on or after cycle 2 (safety population) of any grade were reported in 91.3% of R-SC and 90.4% R-IV patients (P = 0.755). Grade 3 or higher AEs on or after cycle 2 were then summarized:
|
% of patients |
R-SC plus CHOP (n=369) |
R-IV plus CHOP (n=188) |
p-value |
|---|---|---|---|
|
Any grade ≥3 AE |
58.3 |
54.3 |
0.368 |
|
Neutropenia |
21.7 |
18.1 |
0.374 |
|
Neutrophil count decreased |
12.7 |
13.3 |
0.894 |
|
Febrile neutropenia |
12.5 |
6.9 |
0.058 |
|
WBC count decreased |
7.3 |
5.3 |
0.472 |
|
Lymphocyte count decreased |
5.4 |
4.8 |
0.842 |
|
Pneumonia |
5.4 |
2.1 |
0.080 |
|
Anemia |
4.3 |
3.7 |
0.824 |
|
Platelet count decreased |
3.0 |
1.6 |
0.402 |
|
Leukopenia |
1.9 |
2.7 |
0.550 |
SAEs in R-SC and R-IV patients included febrile neutropenia (11.7% vs. 6.4%), pneumonia (6.8% vs. 3.2%), and neutropenia (3.5% vs. 4.8%). A similar proportion of patients in each group discontinued rituximab due to AEs or infections. Lugtenburg also presented data on Administration Related Reactions (ARRs) that occurred in ≥2% of patients. Injection-site reactions were reported in 5.7% of R-SC patients versus 0 R-IV patients (P < 0.001). Fatigue occurred in 1.9% and 2.1%, and nausea occurred in 2.2% and 2.1%, of R-SC and R-IV patients, respectively. Decreased lymphocyte count was reported in 4.1% and 2.1% of R-SC and R-IV patients, and decreased neutrophil count was observed in 1.4% and 4.3% (P = 0.04), respectively. ARRs of any grade were reported in 20.9% of R-SC and 21.3% of R-IV patients, and grade ≥3 ARRs occurred in 2.7% of R-SC and 4.8% R-IV patients.
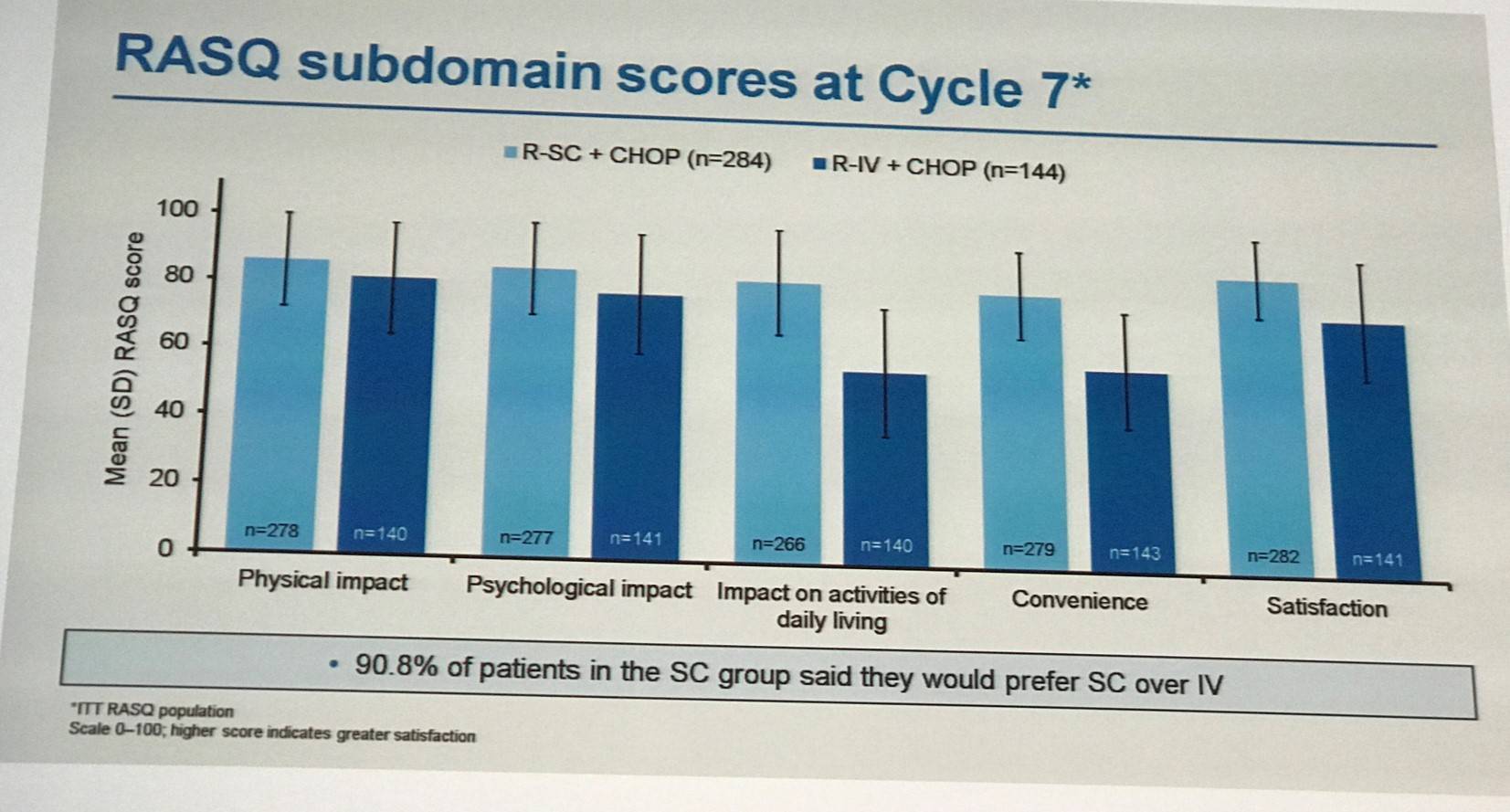
In terms of time saving, median administration times were shorter for R-SC (6 minutes) compared to R-IV (2.6–3.0 hours). From cycle 2 onwards, 27–56% of patients receiving R-SC spent less than 2 hours in a chair/bed receiving rituximab compared to <1–5% of patients receiving R-IV.
Lugtenburg concluded the talk by stating that R-SC and R-IV demonstrated similar efficacy in newly diagnosed DLBCL patients. Safety profiles were comparable and no new safety signals were identified. A similar incidence of AARs was found between R-SC and R-IV, but there was a higher incidence of febrile neutropenia in R-SC treated patients. Patient satisfaction, convenience, and impact on daily life were improved with SC administration compared to IV administration. The findings of the phase III MabEase study support the use of R-SC as first-line treatment for DLBCL.
- la Fuente Burguera A. & Dreyling M. Aggressive Non-Hodgkin Lymphoma – 1st 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain.
- Le Gouill S. et al. Rituximab maintenance after autologous stem cell transplantation prolongs survival in younger patients with Mantle Cell Lymphoma: final results of the LyMa trial of the LYSA/GOELAMS group. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract S105].
- Tilly H. et al. Pola-R-CHP: polatuzumab vedotin combined with rituximab, cyclophosphamide, doxorubicin, prednisone for patients with previously untreated Diffuse Large B-Cell Lymphoma. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract S106].
- Lugtenburg P. et al. Rituximab SC and IV plus CHOP show similar efficacy and safety in the randomized MabEase study in first-line DLBCL. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract S107].

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








