All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
iwCLL 2017 | Ibrutinib plus FCG induces a higher rate of MRD negative remission in the bone marrow than FCG alone in patients with newly diagnosed CLL/SLL
Bookmark this article
On 14th May 2017, during iwCLL, the fifth session took place titled “Additional Considerations for the Initial Treatment of CLL.” This session was chaired by Richard Furman (Weill Cornell) and Jae Park (Memorial Sloan Kettering Cancer Center).
Nitin Jain, from the University of Texas MD Anderson Cancer Center, Houston, Texas, USA, gave a presentation titled “Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (GA101) (iFCG) for previously untreated patients with Chronic Lymphocytic Leukemia (CLL) with mutated IGHV and non-del(17p)” during this session.
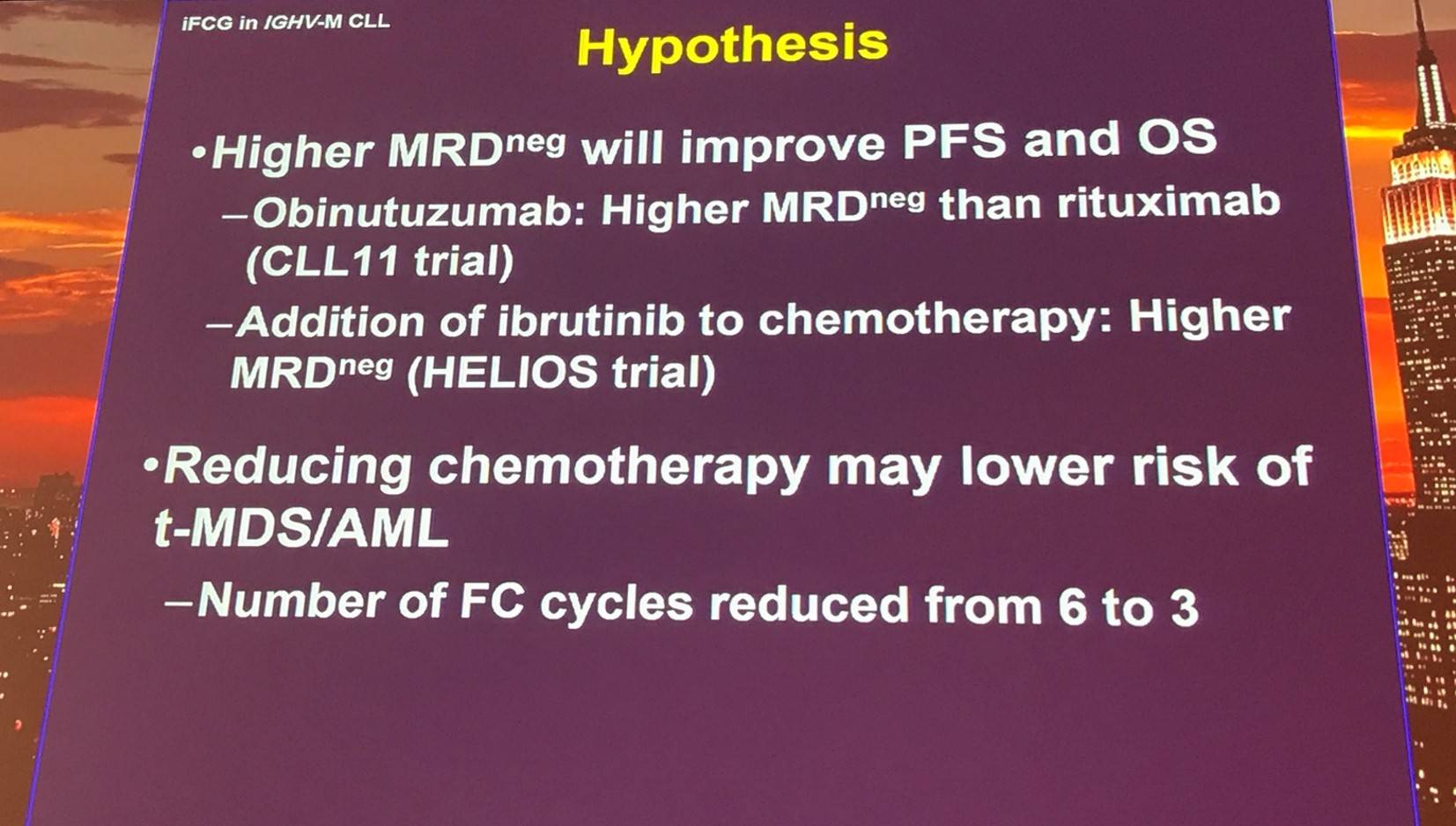
Results of this phase II trial (NCT02629809) were presented; the primary endpoint of the study was CR/CRi with negative MRD in bone marrow.
Eligibility criteria:
- Previously untreated CLL/SLL meeting iwCLL treatment criteria
- Adult patients (≥18 years old)
- Mutated IGHV
- No del(17p) or mutated TP53
- Adequate organ function
- ANC >500/µl
- Platelet >50,000/µl
- ALT and AST ≤2.5 x ULN
- Total bilirubin ≤1.5 x ULN
- GFR ≥30ml/min
Treatment:
- 3 courses of iFCG:
- Ibrutinib 420mg once daily continuously starting C1D1
- Obinutuzumab 100mg C1D1, 900mg C1D2, 1,000mg C1D8, 1,000mg C1D15, 1,000mg C2D1, 1,000mg C3D1
- Fludarabine 25mg/m2 daily for 3 days each course
- Cyclophosphamide 250mg/m2 daily for 3 days each course
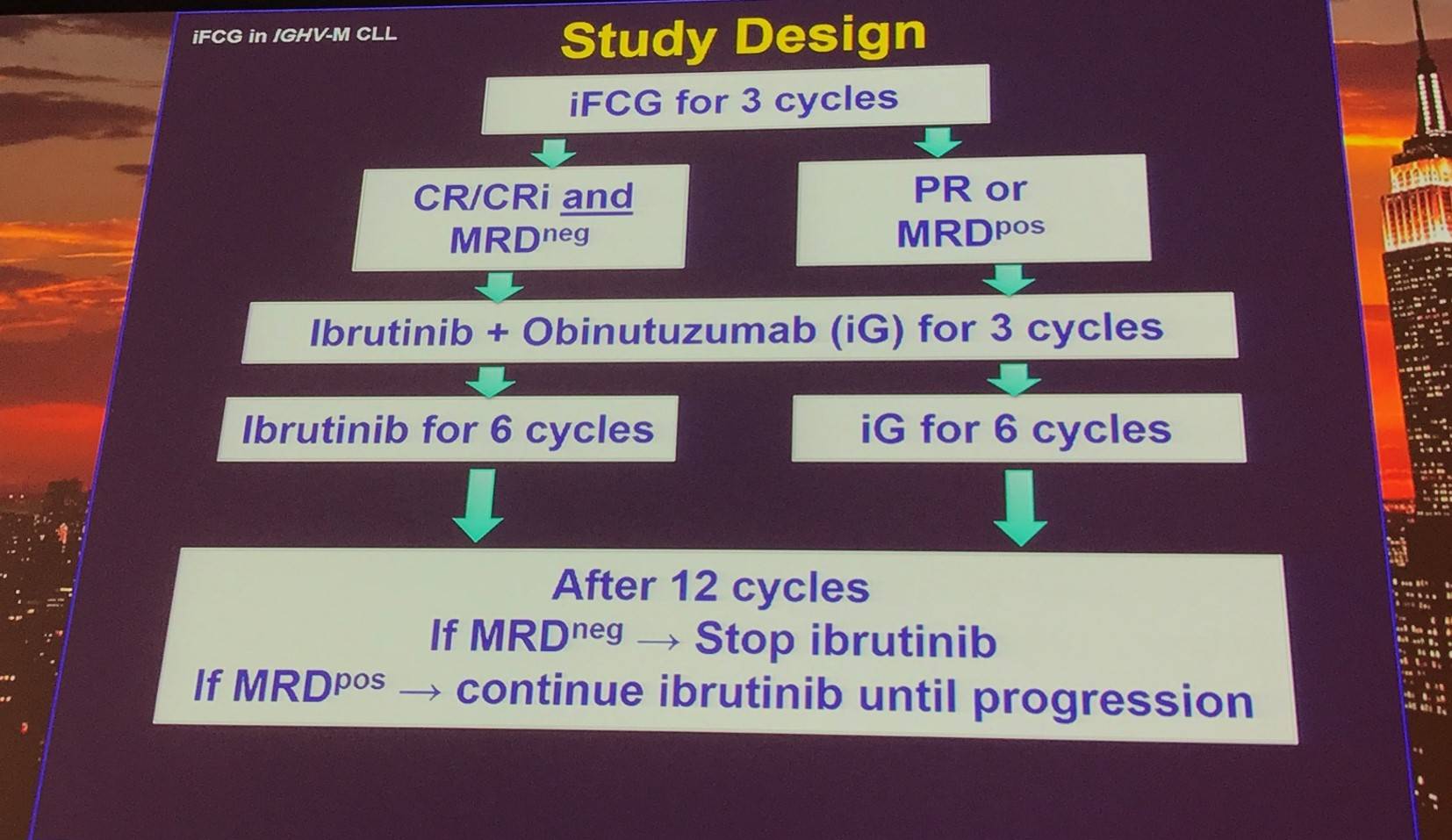
Response evaluation:
Response were measured as outlined by the iwCLL 2008 criteria:
- Blood, marrow, and CT scans: every 3 months during first year, then Q6 months
- Any lymph node >1.5cm on CT: PR
- MRD assessed by 4 color flow cytometry in bone marrow (sensitivity 10-4)
Trial status (9th May 2017):
- The first patients enrolled in April 2016
- 29 patients have initiated treatment
- 24 patients have completed 3 cycles of iFCG (1 off study after 3 cycles due to pulmonary MAC infection)
- 4 patients are receiving iFCG cycles
- 1 patient who received C1D1 obinutuzumab (100mg) and one dose of ibrutinib (420mg) is now off study (grade 3 Infusion Related Reaction [IRR] and grade 4 thrombocytopenia)
- Median follow-up = 8.3 months (range, 0.9–13.3)
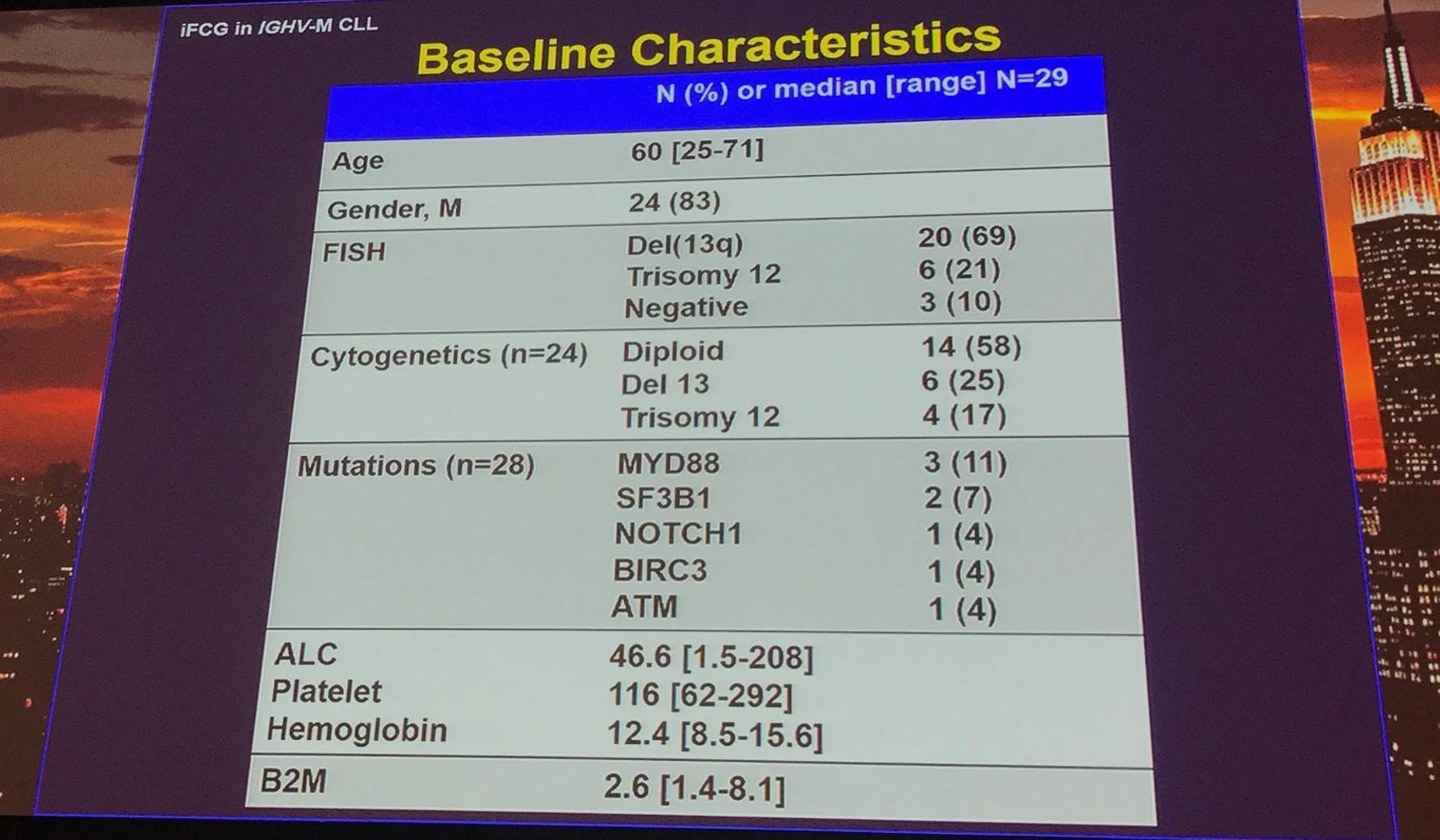
Clinical response:
|
|
3 months |
|
|---|---|---|
|
|
N=24 (%) |
BM MRD (%) |
|
ORR |
24/24 (100) |
20/24 (83) neg |
|
CR/CRi |
10/24 (42) |
All neg |
|
PR |
14/24 (58) |
10/14 (71) neg |
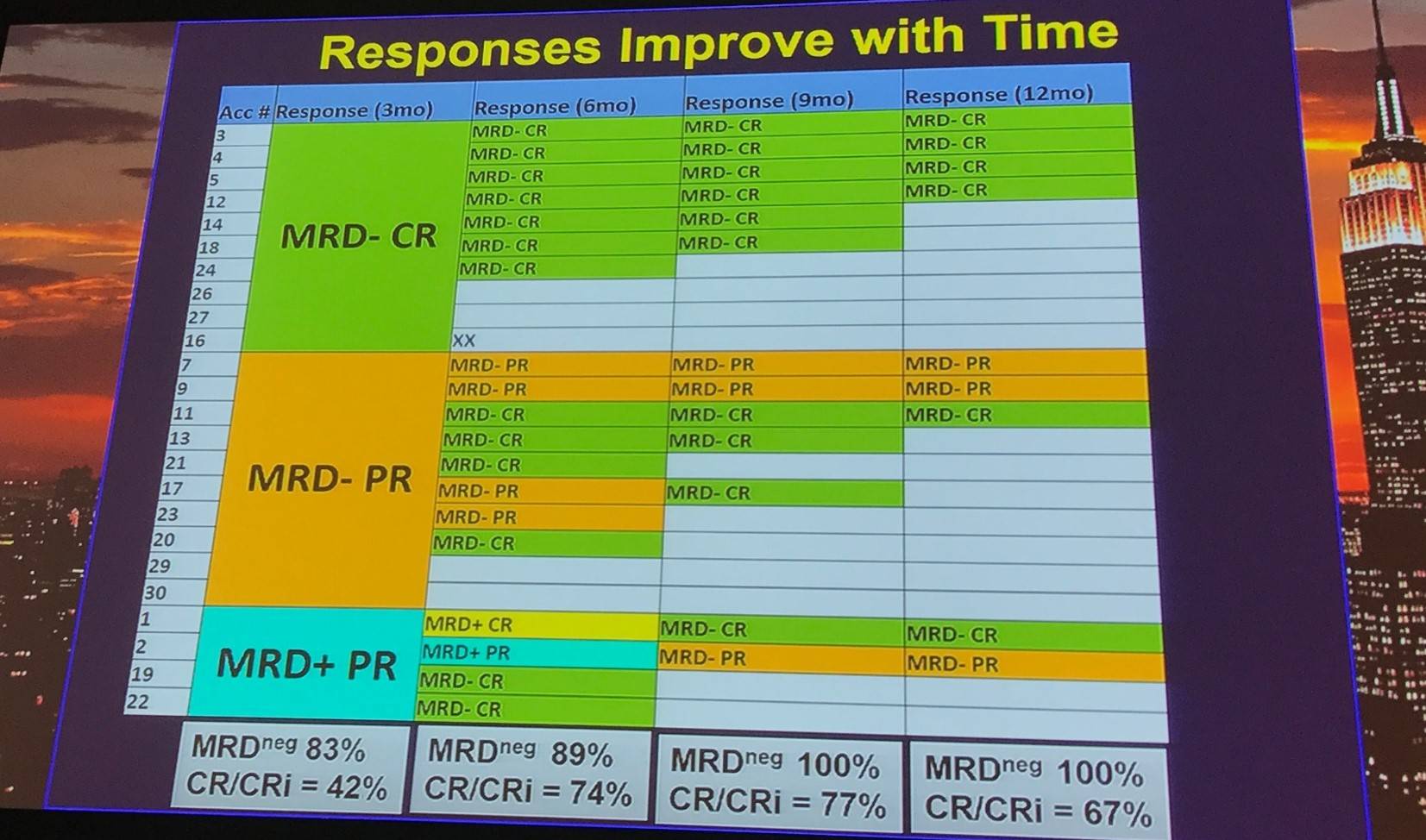
After 3 cycles, a higher proportion of patients treated with iFCG were MRD negative (83%) compared to patients who received FCR (26%).
Moreover, a higher pre-treatment β2M correlated with a lower rate of MRD negativity after 3 cycles of iFCG (P = 0.035):
|
|
n |
BM MRD negativity (%) |
|---|---|---|
|
β2M ≥4 |
6 |
50 |
|
β2M<4 |
18 |
94 |
Toxicities:
|
*9 patients (31%) had grade 2 IRR |
||
|
|
N (%) |
|
|---|---|---|
|
|
G3 |
G4 |
|
Neutropenia |
9 (31) |
12 (41) |
|
Thrombocytopenia |
12 (41) |
1 (3) |
|
ALT/AST |
3 (10) |
1(3) |
|
Atrial fibrillation |
1 (3) |
|
|
Arthralgia |
1 (3) |
|
|
IRR* |
1 (3) |
|
Reported infections included: neutropenic fever (n=4), as well as PCP pneumonia, pulmonary MAC infection, acute cholecystitis, and herpes zoster (n=1 each).
Dose reductions were reported in 57% of patients for FC and 18% of patients for ibrutinib. Treatment delay >2 weeks was reported in 35% of patients (due to thrombocytopenia, transaminitis, and infection).
Nitin Jain finished his talk with a concise conclusion slide:
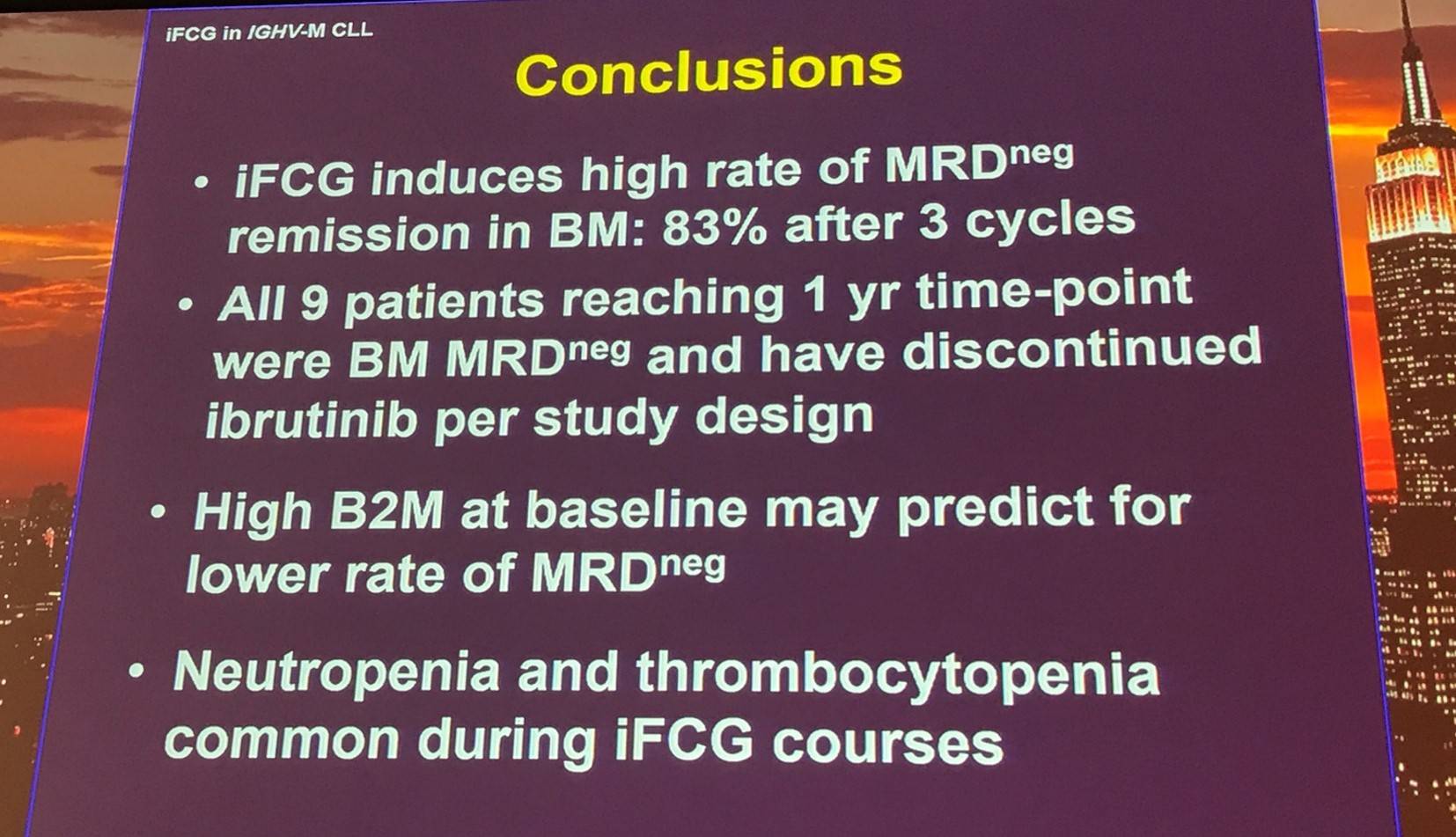
- Jain N. Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (GA101) (iFCG) for previously untreated patients with Chronic Lymphocytic Leukemia (CLL) with mutated IGHV and non-del(17p). XVII International Workshop on Chronic Lymphocytic Leukemia; 2017 May 12–15; New York, USA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








