All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
iwCLL 2017 | Macrophage response to Mycobacterium tuberculosis may be compromised in patients with CLL treated with ibrutinib
Bookmark this article
The second session at this year’s iwCLL was titled “Role of Non-Leukemic Cells and the Microenvironment in CLL Development”, and was jointly chaired by Silvia Deaglio (University of Turin, Torino, Italy) and Christopher Pepper (Cardiff University, Wales, UK).
During this session, Mercedes Borge from the National Scientific and Technical Research Council, Buenos Aires, Argentina, gave a talk titled “Effects of ibrutinib on macrophage-polarization and on the immune-response against Mycobacterium tuberculosis.”
Ibrutinib (a BTK inhibitor) is now approved by the US Food and Drug Administration (FDA) as first-line therapy for CLL. Ibrutinib not only effects leukemic B-cells, but has also been reported to effect other cells in the tumor microenvironment and the immune system, macrophages for example (Kohrt et al. 2014; Borge et al. 2015; Fiorcari et al. 2016).
Macrophages are a key component of the innate immune system, defending against pathogens (fungi, extracellular bacteria), but Mycobacterium tuberculosis (Mtb) in particular. Macrophages phagocytose and kill Mtb and also release cytokines (TNF-α) to promote granuloma formation, which prevents the growth and spread of the bacterial infection. It has been reported that patients treated with TNF-α blockers have a 25-fold increased risk of tuberculosis. Moreover, the incidence of tuberculosis differs greatly between countries. Argentina and Brazil, where ibrutinib has recently approved, have higher incidences (up to 12 times) of tuberculosis than in the US.
The work presented during this talk aimed to explore the effect of ibrutinib on macrophage polarization and on the effector response to Mtb.
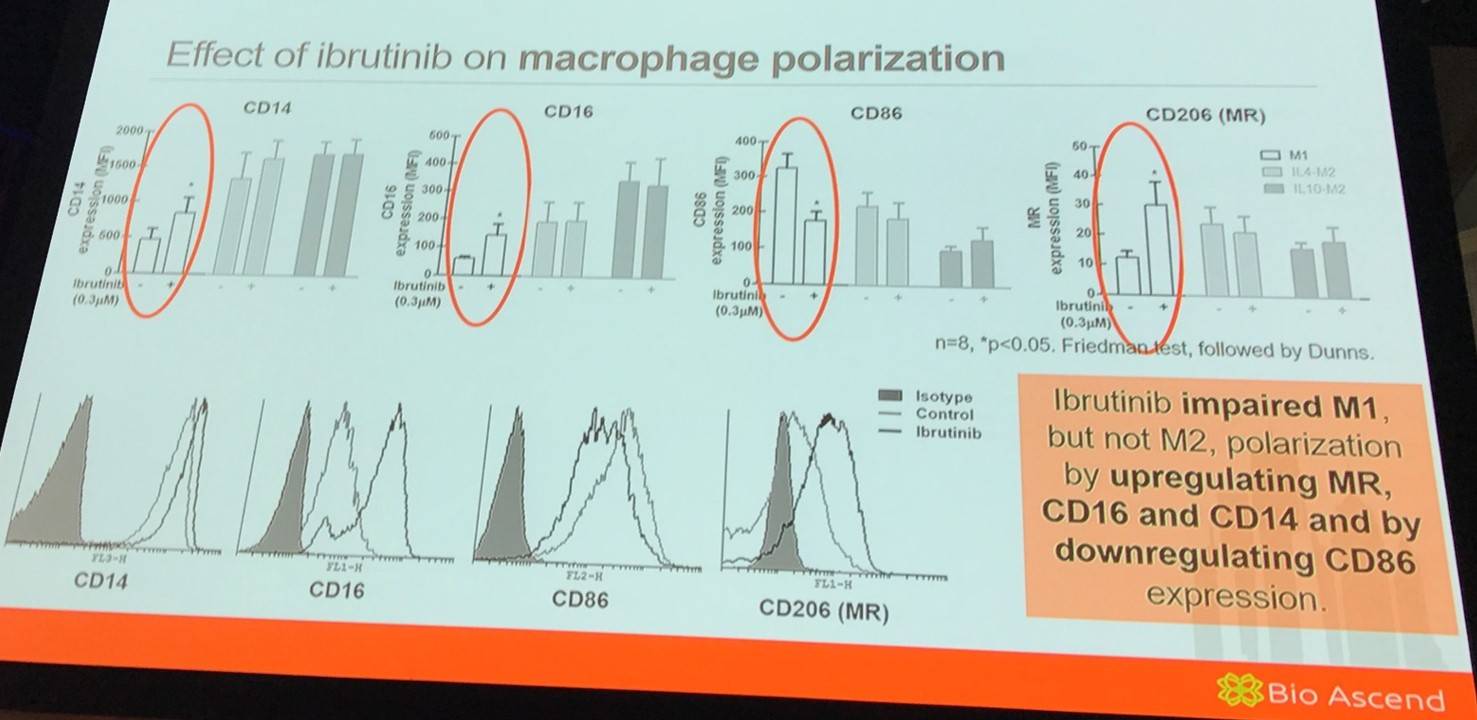
It has been previously reported that M1 cells are less motile in a 3D environment than M2 cells. Therefore, the group aimed to assess if ibrutinib could affect M1 migration in a 3D matrigel matrix. It was found that ibrutinib increased M1 migration in matrigel compared to control cells.
Additionally, it was found that ibrutinib did not modulate STAT1 (involved in IFN-γ-mediated M1 polarization) or STAT6 (involved in IL-4-mediated M2 polarization) activation during macrophage polarization.
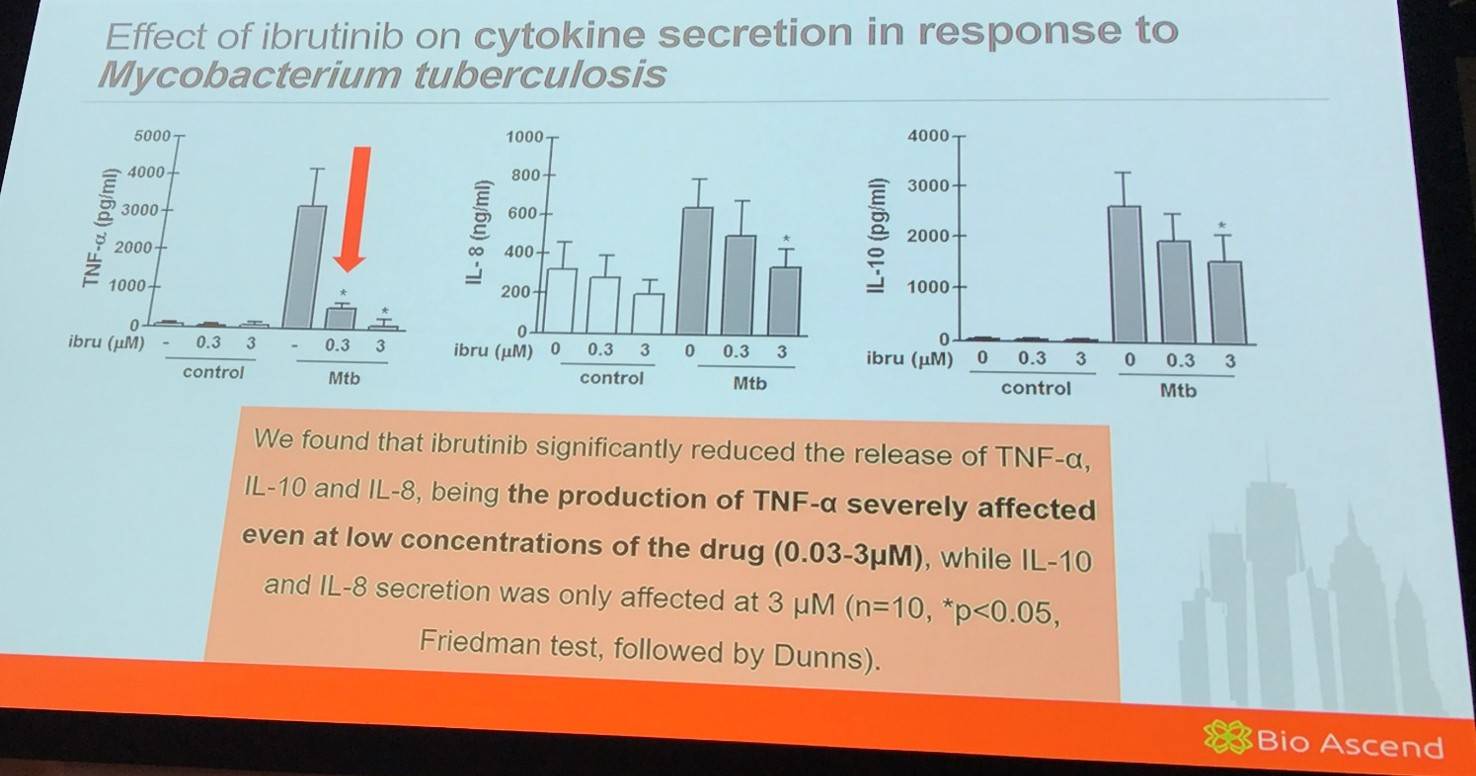
Moreover, it was found that ibrutinib significantly induced release of TNF-α in response to TLR2 and TLR4 stimulation even at low concentrations.
Mercedes Borge summarized her talk with a concise conclusion slide:
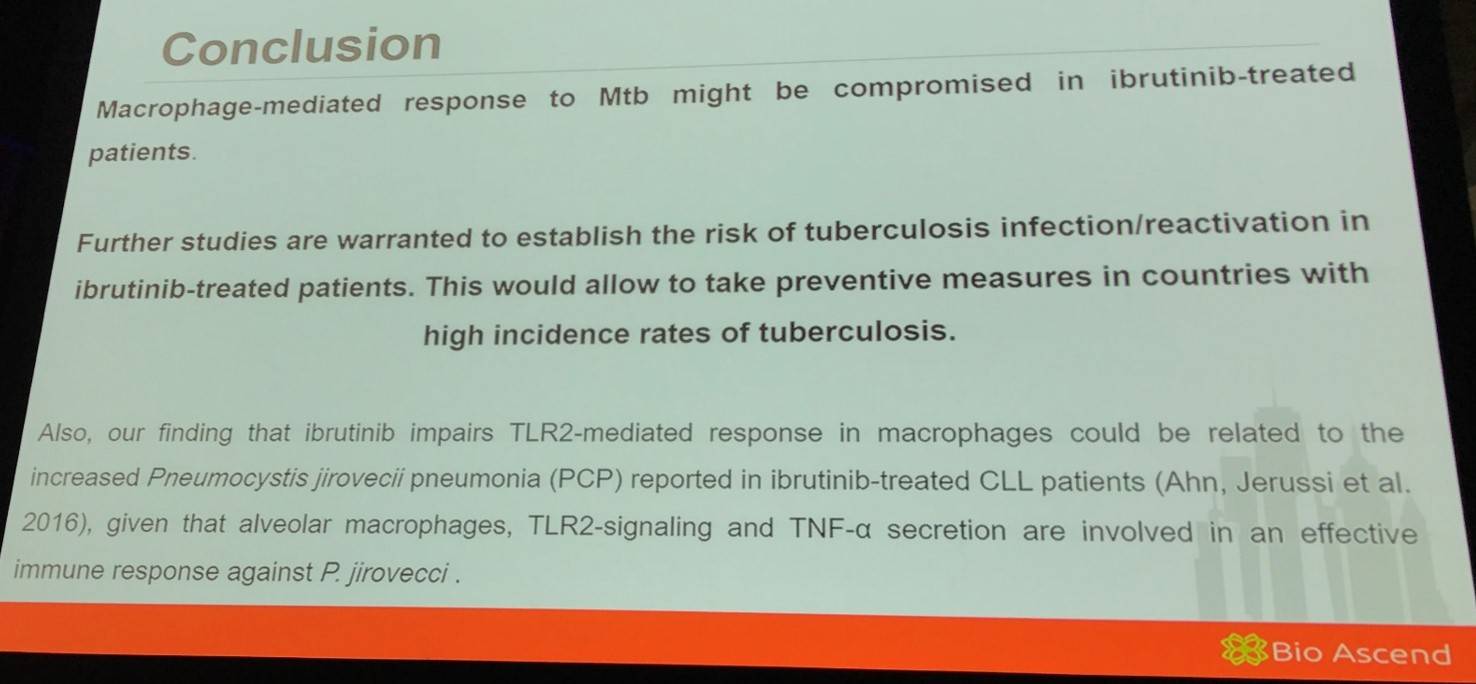
- Borge M. Effects of ibrutinib on macrophage-polarization and on the immune-response against Mycobacterium tuberculosis. XVII International Workshop on Chronic Lymphocytic Leukemia; 2017 May 12–15; New York, USA.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








