All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
An expert panel hosted by

Sequencing immune-based therapies in B-cell malignancies
with Ulric Jäger, Sagar Lonial, and Krina Patel

Saturday, June 15 | 18:00-19:30 CEST
Register nowThis independent education activity is sponsored by Bristol Myers Squibb. All content is developed independently by the faculty. Funders are allowed no direct influence on the content of this activity.
The Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Promising results of a new HDAC inhibitor, entinostat in phase II (ENGAGE-501)
Bookmark this article
Batlevi C. L. from the Memorial Sloan Kettering Cancer Center (MSKCC) and colleagues reported a phase II of entinostat in 49 heavily pre-treated Hodgkin Lymphoma (HL) patients. This study was published in Haematologica in August 2016.
They treated 33 patients with oral entinostat once every other week (schedule A) and 16 patients once weekly in 3 of 4 weeks (schedule B). Their key findings were:
- ITT ORR = 12%
- ITT Disease control (CR + PR + SD >6 months) = 24% (27% schedule A and 19% schedule B)
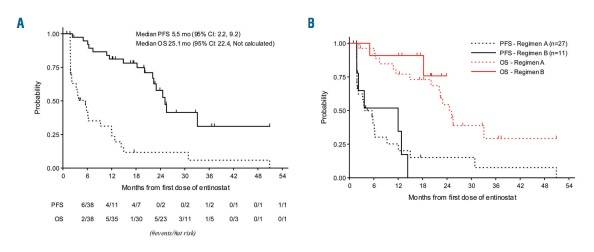
- Median follow-up of 27.9 months: mPFS = 3.8 months and mOS = 24.6 months
- The main toxicity was hematologic with the most common grade 3-4 AEs being thrombocytopenia (63%), anemia (47%), neutropenia (41%), leukopenia (10%) and extra-hematological toxicity (hypokalemia and hypophosphatemia) was <10%
- Dose modifications (decrease or delay) were performed in 51% patients
Conclusions
The results of the phase II ENGAGE-501 demonstrated clinical efficacy and a manageable toxicity profile of entinostat, a new HDAC inhibitor, in a heavily pre-treated HL population. These results seem encouraging for future treatment combinations of HDAC inhibitors with checkpoint inhibitors and have highlighted a potential synergistic effect between the two drug classes.
Abstract
ENGAGE- 501: phase II study of entinostat (SDNX-275) in relapsed and refractory Hodgkin lymphoma
Connie Lee Batlevi et al.
Classical Hodgkin lymphoma treatment is evolving rapidly with high response rates from antibody-drug conjugates targeting CD30 and immune checkpoint antibodies. However, most patients do not achieve a complete response, therefore development of novel therapies is warranted to improve patient outcomes. In this phase II study, patient with relapsed or refractory Hodgkin lymphoma were treated with entinostat, an isoform selective histone deacetylase inhibitor. Forty-nine patients were enrolled: 33 patients on Schedule A (10 or 15 mg oral entinostat once every other week); 16 patients on Schedule B (15 mg oral entinostat once weekly in 3 of 4 weeks). Patients received a median of 3 prior treatments (range 1-10), with 80% of the patients receiving a prior stem cell transplant and 8% of patients receiving prior brentuximab vedotin. In the intention-to-treat analysis, the overall response rate was 12% while the disease control rate (complete response, partial response, and stable disease beyond 6 months) was 24%. Seven patients did not complete the first cycle due to progression of disease. Tumor reduction was observed in 24 of 38 (58%) evaluable patients. Median progression-free survival and overall survival was 5.5 and 25.1 months, respectively. The most frequent grade 3 or 4 adverse events were thrombocytopenia (63%), anemia (47%), neutropenia (41%), leukopenia (10%), hypokalemia (8%), and hypophosphatemia (6%). Twenty-five (51%) patients required dose-reductions or delays. Pericarditis/pericardial effusion occurred in one patient after 12 cycles of therapy. Future studies are warranted to identify predictive biomarkers for treatment response and to develop mechanism-based combination strategies. (clinicaltrials.gov identifier:00866333)
- Batlevi C. L. et al. ENGAGE- 501: phase II study of entinostat (SNDX-275) in relapsed and refractory Hodgkin lymphoma. Haematologica. 2016 Aug;101(8):968-75. doi: 10.3324/haematol.2016.142406. Epub 2016 May 5.

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








