All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
An expert panel hosted by

Sequencing immune-based therapies in B-cell malignancies
with Ulric Jäger, Sagar Lonial, and Krina Patel

Saturday, June 15 | 18:00-19:30 CEST
Register nowThis independent education activity is sponsored by Bristol Myers Squibb. All content is developed independently by the faculty. Funders are allowed no direct influence on the content of this activity.
The Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
TRANSCEND CLL 004 trial: efficacy and safety of liso-cel in patients with R/R CLL or SLL
Bookmark this article
The Lymphoma Hub is pleased to present a visual abstract summarizing the latest results from TRANSCEND CLL 004 (NCT03331198).1
Despite advancements in the therapeutic landscape, there remains an unmet need for patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), especially after progression from Bruton’s kinase inhibitors (BTKi) or venetoclax failure.1 In the phase I/II TRANSCEND CLL 004 dose-escalation study, lisocabtagene maraleucel (liso-cel) was investigated at two doses in patients with R/R CLL or SLL and a subset of patients who had progressed after BTKi and failed venetoclax treatment.1
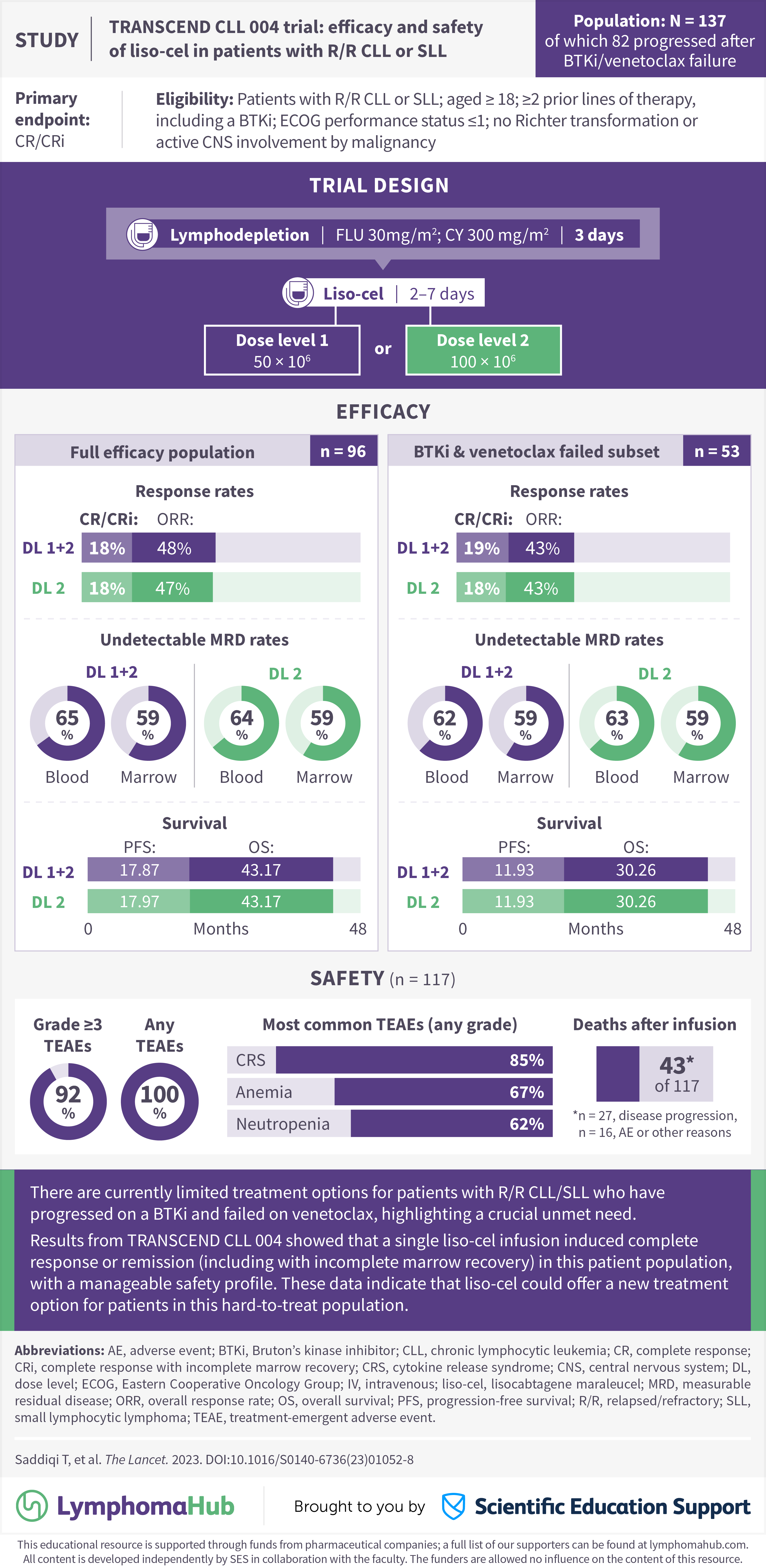
- Siddiqi T, Maloney D, Kenderian S, et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1–2 study. The Lancet. 2023;402:641-654. DOI: 1016/S0140-6736(23)01052-8

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








