All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
EHA 2017 | Treatment of HL, a new staging system for WM, and rituximab monotherapy for sMZL – Clinical Oral Presentation Session
Bookmark this article
An Oral Presentation Session on clinical findings in Hodgkin (HL) and indolent Non-Hodgkin Lymphoma (iNHL) took place on Saturday 24th June during the 22nd congress of the European Hematology Association (EHA). The session was jointly chaired by Andreas Engert, MD, from the University Hospital of Cologne, Germany, and Anton Hagenbeek, MD, PhD, from the University of Amsterdam, The Netherlands.
Abstract S412
The first abstract was given by A. Engert and focused on full results after extended follow-up of the multi-cohort, multicenter, phase II CheckMate 205 trial (NCT02181738). These results were referred to by Anas Younes during the Educational Session on Immunotherapy for Lymphoma; read more on this session here.
Patients with Classical HL (cHL) often harbor 9p24.1 alterations and overexpression of PD-L1 and PD-L2. Nivolumab is a fully humanized IgG4 monoclonal antibody targeting the PD-1 receptor checkpoint pathway.
The phase II CheckMate 205 trial included patients with Relapsed/Refractory (R/R) cHL after Autologous Stem Cell Transplant (ASCT). Initial data from this trial indicated high Objective Response Rates (ORR), promising Duration of Response (DoR), and an acceptable toxicity profile (Younes et al. Lancet Oncol. 2016). Durable responses are valuable in patients with Progressive Disease (PD) after failure of ASCT due to their limited therapeutic options. Nivolumab was administered at 3mg/kg IV Q2W until PD or unacceptable toxicity. Patients could also choose to discontinue nivolumab and proceed to Allogeneic Stem Cell Transplant (allo-HCT). The primary endpoint was ORR by Independent Review Committee (IRC) and additional endpoints included CR/PR rate, PFS by IRC, OS, and safety. Three cohorts of patients were enrolled:
- Cohort A (n=63): Brentuximab vedotin (BV) naïve, extended median follow-up of 19 months
- Cohort B (n=80): BV after ASCT, extended median follow-up of 23 months
- Cohort C (n=100): BV before and/or after ASCT, extended median follow-up of 16 months
The demographics of the patients of the three cohorts were summarized in a table:
|
|
Cohort A |
Cohort B |
Cohort C |
Overall |
|---|---|---|---|---|
|
Age, years |
33 (18–65) |
37 (18–72) |
32 (19–69) |
34 (18–72) |
|
Male, % |
54 |
64 |
56 |
58 |
|
ECOP PS, % 0 1 |
62 38 |
53 48 |
50 50 |
54 46 |
|
Disease stage at study entry, % IV |
38 |
68 |
61 |
57 |
|
Previous lines of therapy Prior radiotherapy, % |
2 (2–8) 59 |
4 (3–15) 74 |
4 (2–9) 69 |
4 (2–15) 68 |
|
Time from diagnosis to first dose of nivolumab, years |
3.1 (1.0–30.6) |
3.4 (0.2–19.0) |
1.7 (0.2–17.0) |
2.0 (0.2–19.0) |
|
Time from ASCT to first dose of nivolumab, years |
1.0 (0.3–18.2) |
3.4 (0.2–19.0) |
1.7 (0.2–17.0) |
2.0 (0.2–19.0) |
|
Patients still on treatment, % |
48 |
40 |
35 |
40 |
Following this, Engert summarized the safety outlined after the extended follow-up:
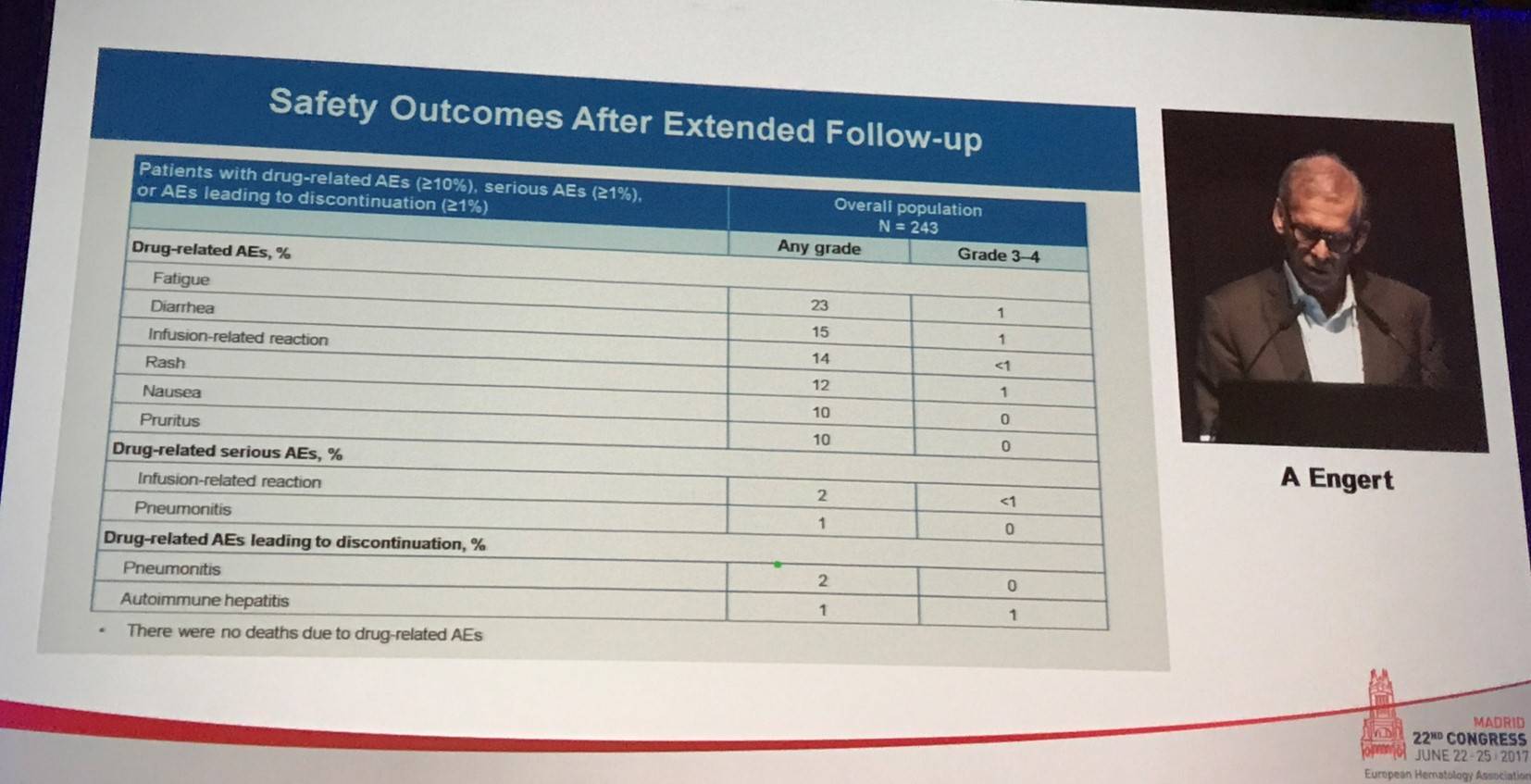
Overall, more than 95% of evaluable patients demonstrated a decrease in tumor burden. In all patients (n=243), ORR per IRC was 69% (95% CI, 63– 75%), with a CR rate of 16%, PR rate of 53%. Stable Disease (SD) was achieved in 19%, and PD was reported in 9%. Per investigator assessment, one-third of patients achieved CR and 39% achieved PR. In post-hoc analyses, responses were similar irrespective of BV treatment sequence. In cohorts A, B, and C, ORR per IRC was 65%, 68%, and 73%. Median DoR in responders overall was 17 months (range, 13–20); in those who achieved CR median DoR was 20 months (range, 16–NE). Median DoR in responders in cohorts A, B, and C was 20, 16, and 15 months. Median PFS for all patients was 15 months (95% CI, 11–19); for cohorts A, B, and C, this was 18, 15, and 12 months, respectively. 12-month OS in all patients was 92% (95% CI, 88–95) and in cohorts A, B, and C was 93%, 95%, and 90%. Responses according to refractory status were also presented by Engert:
|
|
Primary refractory (n=142) |
Refractory to last line (n=114) |
Refractory to BV after ASCT (n=70) |
|---|---|---|---|
|
Objective response, % |
73 |
68 |
69 |
|
Best overall response, % CR PR |
18 55 |
13 54 |
6 63 |
|
Median DoR in patients with PR, months (95% CI) |
13 (9–18) |
17 (9–NE) |
17 (8–NE) |
A fourth cohort has been planned for the CheckMate 205 trial with an estimated enrolment of 50 patients with newly diagnosed advanced stage cHL. These patients will receive around 8 weeks of monotherapy with nivolumab (240mg IV Q2W [4 doses]) then will receive approximately 22 weeks of combination therapy of nivolumab (240mg IV) with doxorubicin, vinblastine, and dacarbazine (AVD) Q2W for 6 cycles (12 doses). The primary endpoint will be safety and tolerability measured by incidence of grade 3–5 treatment-related Adverse Events (AEs). Additional endpoints include discontinuation rate, CR and ORR by IRC, PFS, and OS.
Engert concluded this abstract by stating that, with the longest phase II follow-up of a checkpoint inhibitor so far, nivolumab demonstrated frequent and durable responses regardless of depth of response, BV treatment history, or refractory status to previous therapies. An acceptable toxicity profile was also observed. Nivolumab therefore presents as an attractive long-term treatment option for a wide range of patients with cHL who progress after ASCT. Ongoing trials assessing nivolumab in combination with other agents, such as BV, are ongoing including the phase I/II SGN35-025 (NCT02572167) and phase III CheckMate 812 (NCT03138499) trials.
Abstract S413
Andrea Gallamini from the A. Lacassagne Cancer Center, Nice, France, gave the second talk which conveyed long-term results from the GITIL/FIL HD 0607 trial (NCT00795613).
In ABVD-treated, advanced-stage classical Hodgkin Lymphoma (cHL) patients, FDG-PET after two cycles of chemotherapy (PET2) is a powerful predictor of treatment outcome. Early data indicated that adapting treatment according to PET2 could increase the efficacy of standard ABVD.
HD 0607 was a prospective clinical trial that included advanced-stage (IIB–IVB) cHL patients, who were treated with 2 courses of ABVD after which PET2 was performed. PET2 was blindly and independently reviewed using the Deauville 5-point scale (5-PS). PET2+ patients (5-PS score of 4–5) were randomly assigned to BEACOPP with or without rituximab (R). PET2- patients (5-PS score 1–3) continued ABVD treatment with 4 more cycles and when CR was reached, were randomly assigned to either consolidation radiotherapy (Rxt) on the sites of initial Large Nodal Mass (LNM: diameter >5cm) or No Further Treatment (NFT).
Median follow-up for patients completing treatment was 1,303 days (range, 2–2,857). Patients had a median age of 31 years (range, 14–60) and 90.4% had a WHO activity index of 0–1. The proportion of patients with Ann Arbor stage II, III, and IV was 35.7%, 32.2%, and 32.1%. Upon IRC review, 630 patients (81%) were found to be PET2- and 150 (19%) were PET2+.
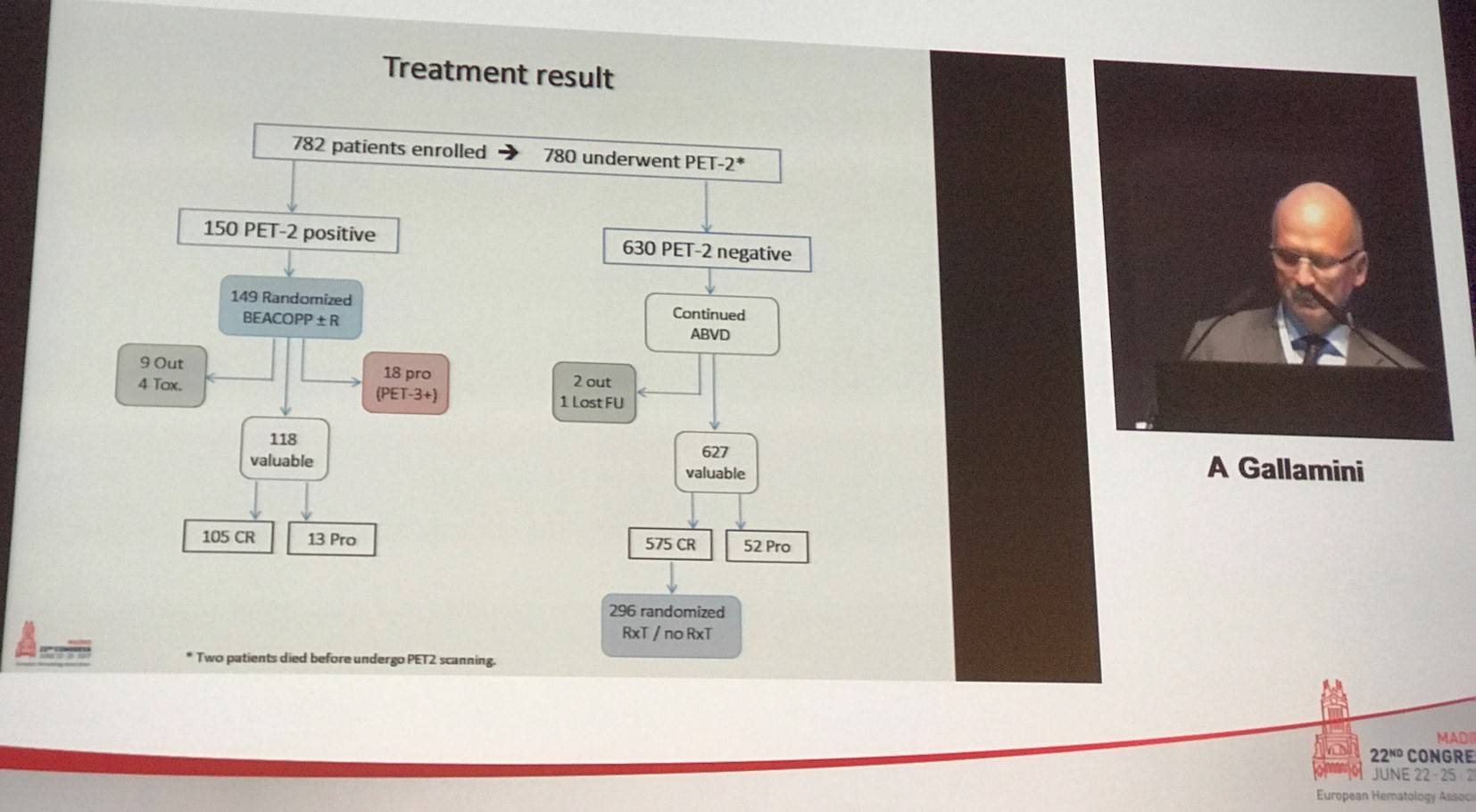
For the entire cohort (n=782), 4-year OS was 96% (95% CI, 94–97%) and 4-year PFS was 83% (95% CI, 80–86%). In PET2- and PET2+ patients, 4-year OS was 97% versus 89%, respectively (P < 0.0001). 4-year PFS was also higher in PET2- patients at 87% compared to 69% in PET2+ patients (P < 0.0001). Survival rates in PET2+ patients randomized to BEACOPP or R-BEACOPP were similar (4-year OS, 89% vs. 90%, P = 0.8950; 4-year PFS, 69% vs. 68%, P = 0.9731). Survival outcomes were also similar in PET2- patients randomized to either Rxt or NFT: 4-year OS was 100% versus 98% (P = 0.0790) and 4-year PFS was 96% versus 93% (P = 0.2882), respectively. 4-year OS and PFS according to Deauville score were also presented. In patients with a 5-PS score of 4, 4-year OS was 92% compared to 83% in patients with a 5-PS of 5 (P = 0.0696). 4-year PFS in patients with 5-PS of 4 or 5 was 81% versus 46%, respectively (P < 0.0001).
In patients treated with ABVD, the incidence of severe (grade 3–4) hematologic, infectious, and pulmonary AEs was 36%, 1%, and 2% compared to 76%, 10%, and 1% in patients who received BEACOPP. Overall there were 30 deaths during the study; 19 from PD, 5 related to SCT, 4 due to infection, 1 case of pulmonary fibrosis, and 1 due to cardiac failure. Two patients died pre-PET, 12 were PET2- and 16 were PET2+.
Gallamini concluded his portion of the session by stating that interim-PET response adapted therapy in patients with advanced HL had a superior long-term disease control versus patients who received standard ABVD therapy. The addition of rituximab did not improve outcome in PET2+ patients switching to BEACOPP. Furthermore, consolidation radiotherapy in the region of LNM did not improve responses. PET2+ patients with a 5-PS score of 4 switching to BEACOPP have a 3-year PFS similar to the entire cohort of patients (4-year PFS, 81%). Patients with a 5-PS score of 5, a much smaller subset of patients, still represent an unmet therapeutic need that require alternative treatment options.
Abstract S414
The third talk in this session included data from a German Hodgkin Study Group (GHSG) analysis of disease characteristics and survival after third recurrence of cHL, and was presented by Paul J. Bröckelmann, MD, University Hospital of Cologne, Cologne, Germany.
Novel agents are altering the therapeutic landscape for cHL and are usually investigated in patients with R/R disease after numerous previous lines of treatment. Compared to 1st–3rd line cHL, disease characteristics and treatment outcomes for 3rd–4th line are poorly documented and understood.
The study presented by Bröckelmann aimed to determine a historical control group of patients for comparison of safety and efficacy of novel agents. Patients with 3 or more tumor-related events (i.e. 3rd relapse) were retrospectively identified in the GHSG database.
Overall, 12,584 patients treated in upfront trials and 449 patients treated in relapsed trials were screened in the GHSG database; 69 patients with 3rd relapse were identified with a median age of 36.6 years (range, 19.8–78.7). Four patients (6%) were aged 65 years or older. The median number of chemotherapies prior to 3rd relapse was 3 (range, 1–3).
Time to Relapse (TTR) was ≤3 months in 22% of patients, 4–12 month in 27% of patients, and >12 months in 51% of patients. Stage I, II, III, and IV disease was observed in 17%, 12%, 13%, and 46% of patients, respectively. ECOG Performance Score was 0, 1, 2, and 3 was seen in 16%, 14%, 4%, and 1% of patients. The proportion of patients who experienced B-symptoms was 16%.
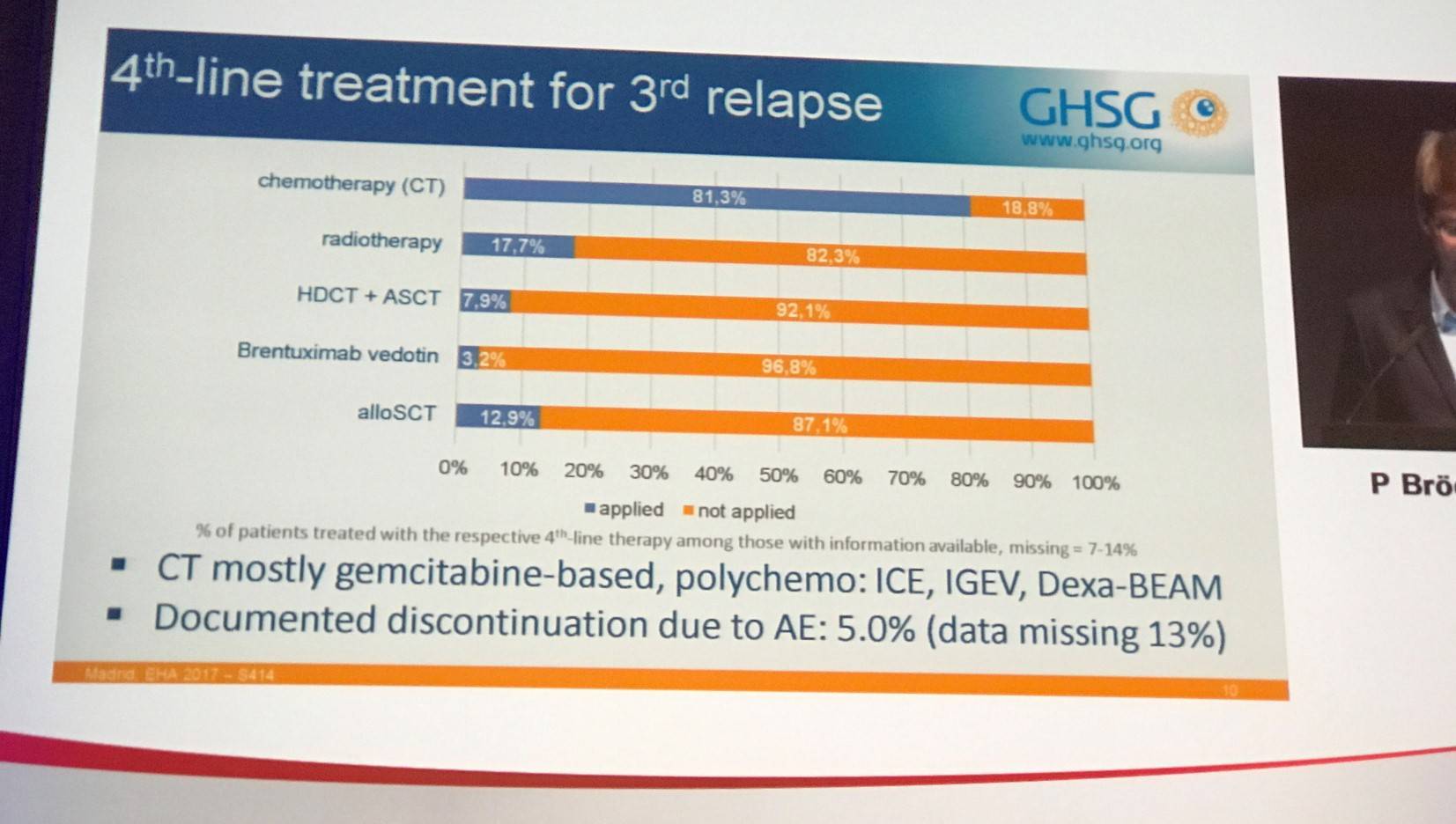
Overall, ORR was 33.3% with 18 patients achieving CR and 5 achieving PR. Nearly one-third (31.9%) of patients progressed. Response was not evaluated in a large proportion of patients (34.8%). The majority of observed patients (87%) had a further PFS event after 3rd relapse. PFS at 18 months was 29.5% (18.5–40.5%), and median PFS was 12.1 months. Nearly two-thirds (65.2%) of observed patients had died after 3rd relapse. OS at 18 months was 65.6% (54.2–77%), and median OS was 36 months.
Bröckelmann also discusses some limitations of the study, which included its retrospective nature and the fact that the analysis only screened patients initially treated in clinical trials so often excluded frail or multi-morbid patients. Less cases were identified than the group expected and hypothesized that patients who experience 2nd relapse or more are underreported. Furthermore, more detailed clinical data regarding disease and treatment characteristics beyond what is included in the database documentation is often unavailable. Bröckelmann also mentioned that the PFS and OS estimated for 3rd line relapse may potentially be “a bit too optimistic.” Despite this, tumor progression and premature death were frequent events.
In summary, until 3rd relapse most patients received HDCT plus ASCT (72.5%). Nearly half (49%) of 3rd relapses developed within 12 months after last therapy and advanced stage disease was frequent in 3rd relapse patients (59% were clinical stage III–IV). 4th line therapy in this patient sample was heterogeneous, but mostly gemcitabine based and rarely administered with curative intent.
Abstract S415
Efstathios Kastritis, MD, from the Greek Myeloma Study Group, Athens, Greece, presented the next talk in this session which focused on revised staging for Waldenström Macroglobulinemia (WM). Kastritis also presented a talk on novel treatment strategies during the WM Educational Session; read more here.
WM is an indolent Lymphoma with a heterogeneous manifestations and may have a prolonged disease course. In some patients however, adverse outcomes and a relatively short disease course has been reported. Therefore, a staging system is needed to offer risk stratification and for use in clinical trials, as well as prognostic information for patients and physicians. A group effort resulted in the creation of the International Prognostic Scoring System for WM (IPSSWM), based on data from patients treated mostly with alkylators and nucleoside analogues and not rituximab. The IPSSWN stratifies patients into 3 different risk groups and is based on 5 covariates: age >65 years, hemoglobin ≤11.5g/dL, platelet counts ≤100x109/L, IgM levels >7.0g/dL, and β2M >3mg/L (Morel et al. Blood. 2009).
|
Stratum |
Score |
5-year OS |
|---|---|---|
|
Low |
0 or 1 (except age) |
87% |
|
Intermediate |
Age or 2 |
68% |
|
High |
≥3 |
36% |
However, the IPSSWM does not account for non-WM related mortality, a common and varied event in patients aged 75 years or older. Moreover, it does not include LDH levels, which is a well-documented prognostic factor of Lymphoma and Multiple Myeloma. Additionally, it does not include serum albumin levels, which has also been shown to have prognostic significance, and the hemoglobin cut-off is higher than what would be considered to define symptomatic disease per IWWM-2 criteria. Moreover, the IPSSWM does not identify very high risk patients.
The aim of the study presented by Kastritis was to revise the current IPSSWM by using a large dataset of symptomatic WM patients treated with different types of primary therapy including rituximab and new agents such as bortezomib. Overall, 492 patients with clinicopathological diagnosis of WM and symptomatic disease were prospectively identified from the database of the Greek Myeloma Study Group. Minimum follow-up was at least 3 years and data was available for β2M, IPSSWM, serum albumin, LDH, and cause of death (by treating physician). The median age of patients was 68.5 years (range 28–92) and 60% were aged over 65 years. Median hemoglobin level was 10.1g/dL and median platelet count was 219x109/L. Median serum albumin and β2M were 3.6g/dL and 3.6mg/L, respectively. LDH >250 IU/L was observed in 16.5% of patients and bone marrow infiltration was seen in 55% of patients.
According to the original IPSSWM applied to this patient cohort, low, intermediate, and high risk patients had 7-year OS of 89%, 65%, and 50%, respectively. Analysis was undertaken to determine the factors associated with early mortality (<3 years). ROC analysis identified albumin <3.5g/dL, β2M >4mg/L, and LDH >250 IU/L as the 3 strongest predictors. Similar results were obtained for ROC analysis of death within 5 years from the start of therapy.
The final multivariate model for the revised IPSSWM was summarized in a series of tables:
|
|
% of patients with the risk feature |
HR (95% CI) |
P value |
|---|---|---|---|
|
Age <65 |
41% |
1 |
|
|
Age 66–75 |
39% |
21. (1.4–2.9) |
<0.001 |
|
Age >75 |
20% |
3.9 (3.0–5.1) |
<0.001 |
|
β2M >4mg/L |
39% |
1.8 (1.3–2.1) |
0.001 |
|
LDH >250 IU/L |
17% |
1.7 (1.2–2.6) |
0.001 |
|
Serum albumin <3.5g/dL |
42% |
1.9 (1.6–2.6) |
<0.001 |
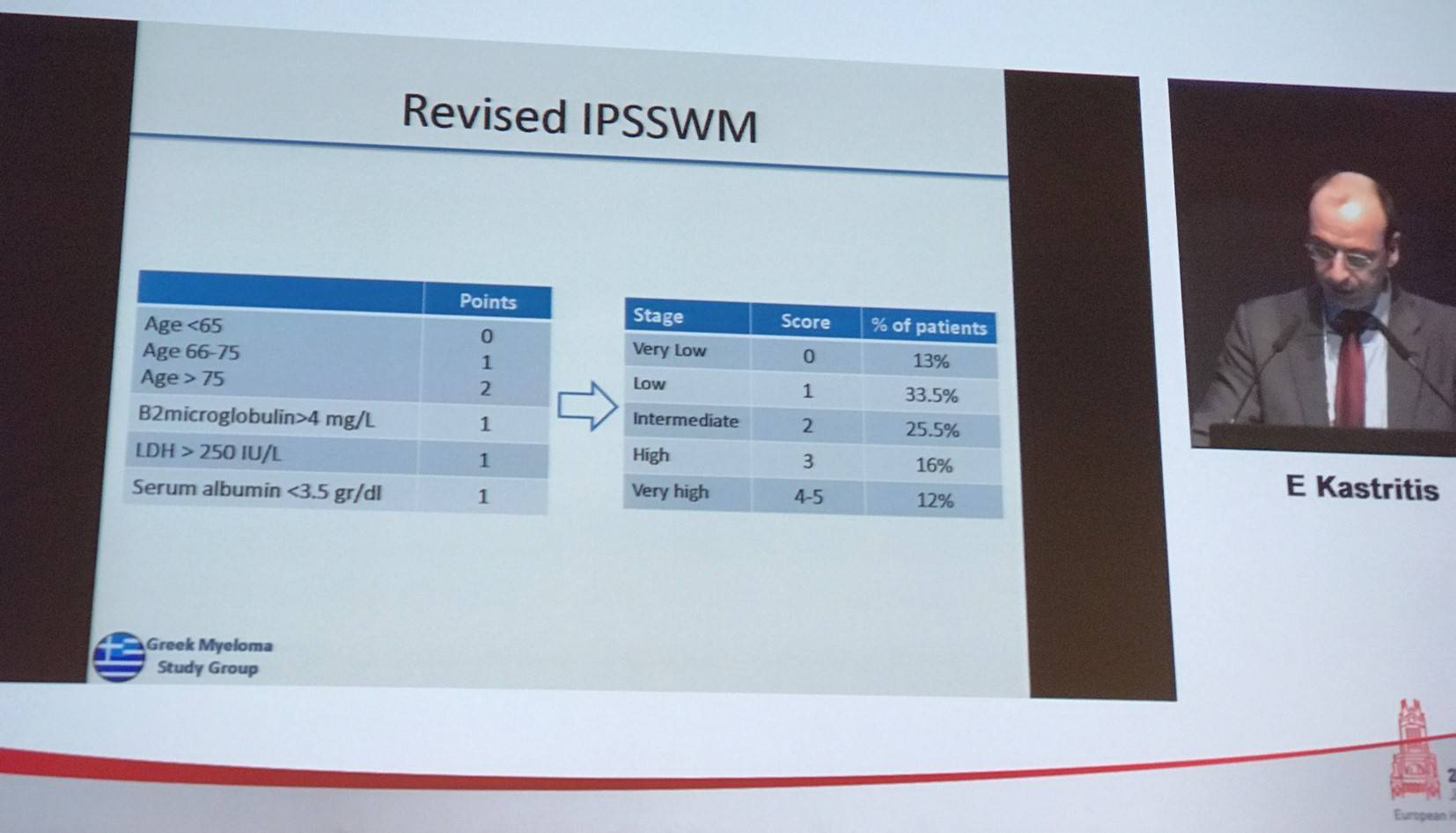
7-year OS in patients stratified by the revised model was 91%, 81%, 58%, 39%, and 22% in very low, low, intermedia, high, and very high risk patients, respectively. Median OS in years in these groups was NR, 10.5, 7.8, 5.9, and 1.6, respectively.
Due to age being a major determinant of disposition, the new model was also evaluated in patients aged >65 years and it retained its prognostic significance. Compared to the original IPSSWM, the new staging system outperformed ISSWM: c-statistics, a measure of performance of a prognostic tool, was 0.652 for IPSSWM (95% CI, 0.627–0.677) vs 0.711 (95% CI, 0.659–0.763) for the new staging system.
Kastritis concluded this talk by stating that the revised staging system is simple to calculate and discriminates groups with very different outcomes. This new staging system requires further validation in an independent cohort.
Abstract S416
The last abstract presented in this session included data from a long-term follow-up study of patients with Splenic Marginal Zone Lymphoma (sMZL) treated with rituximab monotherapy. The talk was given by Christina Kalpadakis from the University of Crete, Heraklion, Greece.
sMZL is a rare B-Cell Lymphoma and demonstrates an indolent course with a median survival of more than 10 years. However, around 10–20% of cases do demonstrate a more aggressive clinical behavior with short survival. There are no reproducible prognostic factors and no standard of treatment.
Previous investigation has found that rituximab monotherapy can be administered successfully in sMZL and can replace splenectomy as first-line treatment.
|
|
Treatment modality |
|||
|---|---|---|---|---|
|
Response and outcome |
Splenectomy (n=1,008) |
Chemotherapy (n=47) |
Rituximab (n=122) |
Rituximab plus chemotherapy (n=123) |
|
ORR (%) |
60–100 |
68–100 |
88–100 |
83–100 |
|
CR (%) |
NA |
20–100 |
31–90 |
54–100 |
|
5-year PFS (%) |
48–61 |
Median: 1.3–4.7 years |
60–73 |
80 |
|
5-year OS (%) |
65–84 |
NR |
70–92 |
86 |
Kalpadakis et al. Best Pract and Res Clin Haematol. 2017.
The study presented by Kalpadakis diagnosed patients based on the WHO criteria. Criteria for initiation of treatment included: bulky/symptomatic splenomegaly, cytopenias, or presence of B-symptoms. All patients received 6 weekly cycles of rituximab as first-line therapy at a dose of 375mg/m2 (induction phase). Splenectomy was not carried out in any patients before rituximab therapy. Maintenance with rituximab at a dose of 375mg/m2 every 2 months for 1–2 years was given according to physician’s discretion in patients who responded to induction. Assessment of response was carried out 2 months after the end of the induction phase and at the end of the maintenance phase.
Overall, 104 consecutive cases were analyzed with a median age of 66 years (range, 41–91). B-symptoms and elevated LDH was observed in 7% and 43% of patients. Thirty percent of patients had anemia, 19% had thrombocytopenia, and 40% had lymphocytosis. Bone marrow infiltration was observed in all patients, and 35% had monoclonal gammopathy.
The Splenic Lymphoma Study Group (SLSG) proposed a risk stratification system separates patients into 3 risk groups with significantly different Lymphoma specific 5-year OS based on 4 variables: presence of extrahilar lymphadenopathy, elevated LDH, low hemoglobin levels, and low platelet counts (Montalban et al. Leuk Lymphoma. 2014). Prognostic classification according to this system was applied to patients in the current study:
|
SLSG prognostic system group |
Number of patients |
% |
|---|---|---|
|
A |
39 |
39 |
|
B |
56 |
56 |
|
C |
5 |
5 |
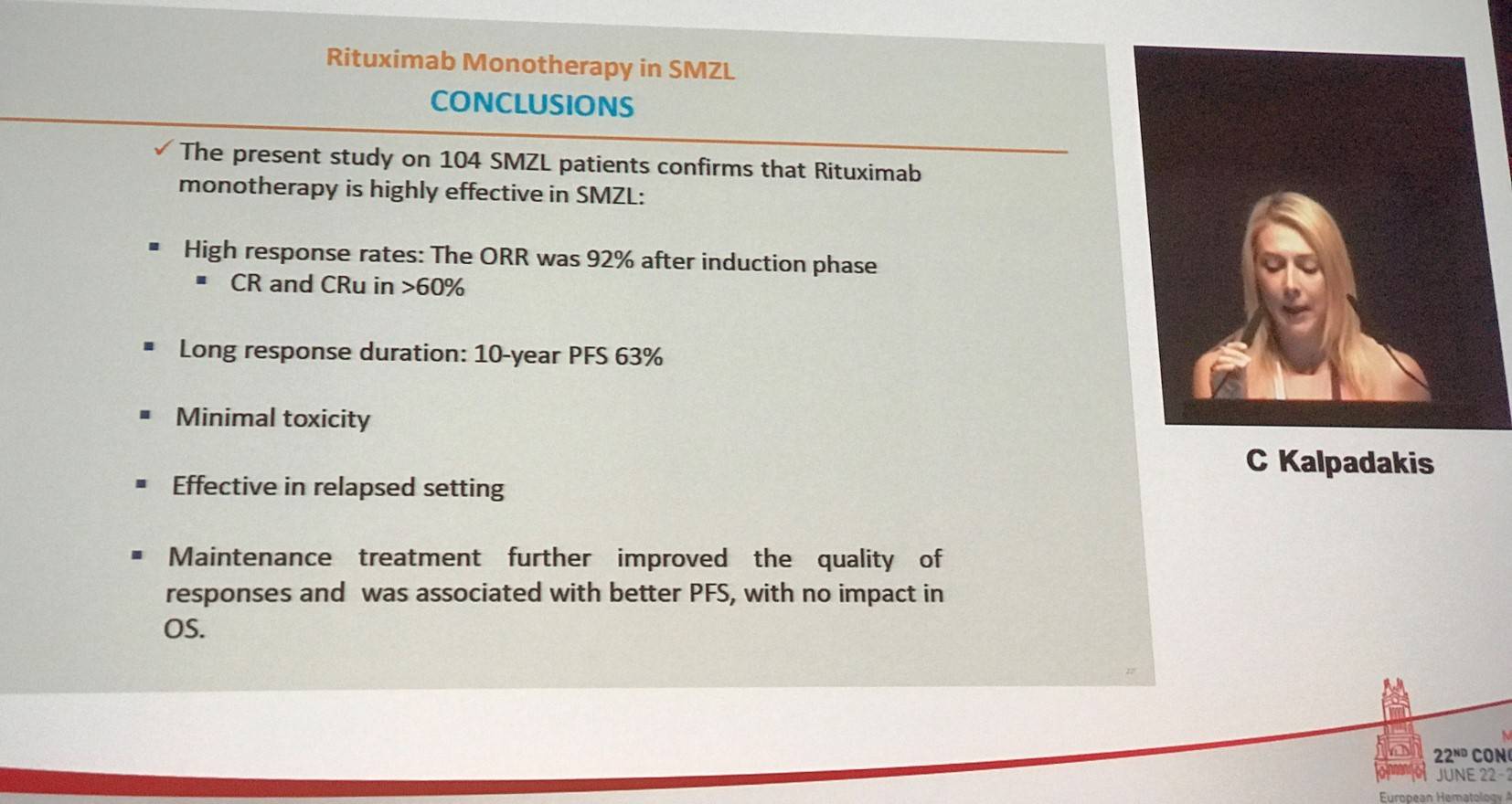
Kalpadakis presented the response rates found at the end of induction phase (n=104): ORR was 92% with a CR rate of 42%, CRu of 22%, PR of 28%, and SD/PD of 8%. The median time to clinical response was 4 weeks (range, 1–48) and the median time to hematological response was 2 weeks (range, 1–32). Response rates at the end of maintenance (n=70) were also presented: CR was 71%, CRu was 19%, PR was 9%, and SD/PD was 1%. PFS for all patients at 5-years and 10-years was 69% and 63%. 7-year PFS in patients who received maintenance was 75% compared to 39% in patients who did not receive maintenance (P < 0.0004). There was no difference in OS between patients who did and did not receive maintenance. Rituximab monotherapy in patients with sMZL resulted in 5-year and 10-year OS of 93% and 85%.
After a median follow-up of 59 months (range, 0.4–172), 22 patients had relapsed and required further therapy. Twelve of these were re-treated with rituximab resulting in 2 CRs, 4 CRus, and 3 PRs. Of the 9 patients that responded, 8 have sustained their second response at the time of this analysis; median duration of 2nd response was 34 months (range, 1–128).
The median time from sMZL diagnosis to histologic transformation was 51 months (range, 32–102). At a median follow-up of 23 months (range, 10–29) after disease transformation: 2/6 patients died due to PD, 3/6 patients are alive and in CR, 1/6 is alive with disease.
Overall, 9 patients have died during the study period: 3/9 were due to unrelated cause in CR, 2/9 from a 2-year malignancy (lung and colon cancer, respectively), 2/9 had histologic transformation to high grade Lymphoma, and 2/9 were due to PD.
In terms of treatment safety, rituximab was well tolerated with no grade 3–4 side effects reported except one patient who developed a serious infusion-related reaction despite adequate pre-medication treatment with steroids, paracetamol, and antihistamines. Two patients experienced reactivation of varicella zoster virus and late onset neutropenia observed in two patients.
Kalpadakis drew her talk to a close with a concise conclusion slide:
- Engert A. & Hagenbeek A. Hodgkin and indolent lymphoma – Clinical. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain.
- Engert A. et al. Nivolumab for relapsed/refractory classical Hodgkin Lymphoma after autologous transplant: full results after extended follow-up of the multicohort multicenter phase II CheckMate 205 trial. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract #S412].
- Gallamini A. et al. Early chemotherapy intensification with escalated BEACOPP in advanced-stage Hodgkin Lymphoma with a positive interim PET-CT after 2 ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract #S413].
- Bröckelmann P.J. et al. Disease characteristics and survival: after 3rd recurrence of Classical Hodgkin Lymphoma: an analysis of the German Hodgkin Study Group. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract #S414].
- Kastritis E. et al. A revised staging system for Waldenström’s Macroglobulinemia. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract #S415].
- Kalpadakis C. et al. Splenic Marginal Zone Lymphoma (sMZL) treated with rituximab (R) monotherapy: a long term follow-up study on 104 patients. 22nd Congress of the European Hematology Association; 2017 June 22–25; Madrid, Spain. [Abstract #S416].

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








