All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Lymphoma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Venetoclax monotherapy for patients with R/R CLL: Results from the phase III VENICE-1 trial
Bookmark this article
Venetoclax combination therapy improves outcomes in patients with relapsed/refractory (R/R) or newly-diagnosed chronic lymphocytic leukemia (CLL).1 Preliminary results from the VENICE II study showed that venetoclax monotherapy improved quality of life (QoL) in patients with R/R CLL. However, there is still a lack of long-term data on venetoclax monotherapy in patients with R/R CLL, including those with previous B-cell receptor kinase inhibitor (BCRi) treatment.1
Here we summarize the results from the VENICE-1 trial (NCT02756611) published by Kater et al.1 in Lancet Oncology.
Study design1
- This was an open-label, single-arm, multicenter phase IIIb trial in adult patients with R/R CLL.
- Patients received an initial dose of 20 mg oral venetoclax once daily, increasing weekly up to 400 mg once daily, for up to 108 weeks.
- QoL was assessed using:
- EuroQol 5 Dimensions 5 Levels Questionnaire (EQ-5D-5L).
- Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) questionnaire.
- Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale
- The primary efficacy endpoint was the complete remission (CR) or CR with incomplete count recovery (CRi) rate in BCRi-naïve patients.
Key findings1
Patients
- In total, 258 patients were included (BCRi-naïve, n = 191; BCRi-pretreated, n = 67).
- Median age was 68 years.
- Median follow-up was 49.5, 49.2, and 49.7 months in the overall cohort, the BCRi-naïve cohort, and the BCRi-pretreated cohort, respectively
Response
- In the BCRi-naïve cohort, the CR/CRi rate at Week 48 was 35% (Figure 1).
- Median duration of response was 25.1, 24.4, and 28.6 months in the overall cohort, the BCRi-naïve cohort, and the BCRi-pretreated cohort, respectively
Figure 1. Response rates at Week 48 in the VENICE-1 trial*
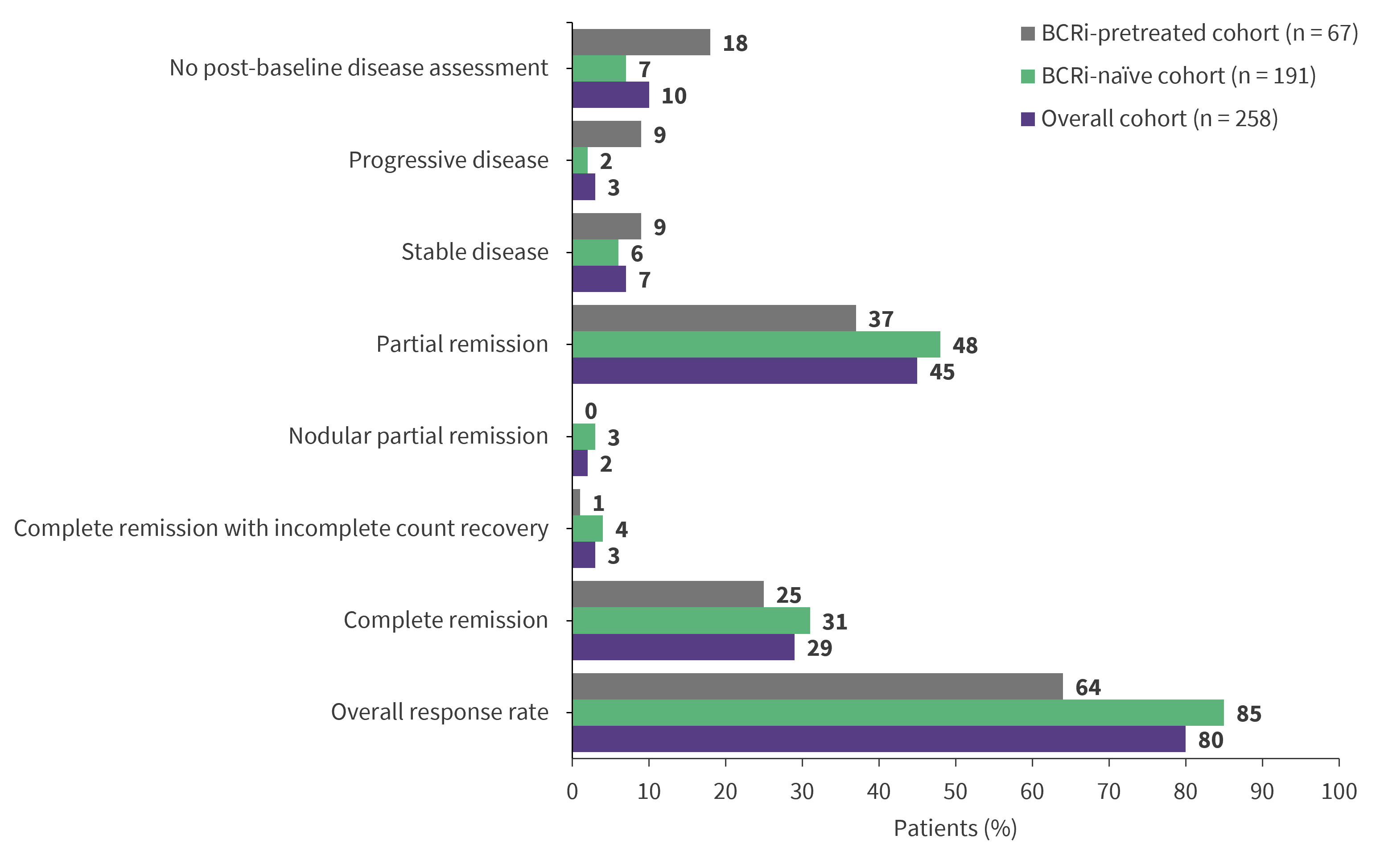
BCRi, B-cell receptor kinase inhibitor.
*Adapted from Kater, et al.1
Survival outcomes
- Median progression-free survival was 28.3, 28.8, and 23.4 months in the overall cohort, the BCRi-naïve cohort, and the BCRi-pretreated cohort, respectively.
- In total, 70 patients died and median overall survival was not reached in the overall, BCRi-naïve, and BCRi-pretreated cohorts.
- The estimated 5-year overall survival rate was 71%, 75%, and 61% in the overall cohort, the BCRi-naïve cohort, and the BCRi-pretreated cohort, respectively.
Quality of life
- Venetoclax treatment was associated with a mean improvement of 8.5 points and 7.1 points in the EQ-5D-5L visual analog score at Weeks 48 and 108, respectively.
- Mean FACT-Leu subscale scores were improved with mean scores of 6.8 and 6.0 at Weeks 48 and 108, respectively.
- Mean FACIT-F scores also improved from baseline by 4.9 points at Week 48 and 3.3 points at Week 108.
Safety
- Treatment-emergent adverse events (TEAEs) of any grade occurred in 98% of patients.
- The most common TEAEs of any grade were neutropenia (43%), diarrhea (39%), and nausea (27%).
- Grade ≥3 TEAEs were observed in 79% of patients.
- The most common Grade ≥3 TEAEs were neutropenia (37%), anemia (13%), and thrombocytopenia (13%).
- Serious TEAEs were reported in 53% of patients.
- The most frequently observed serious TEAEs were pneumonia (8%) and febrile neutropenia (6%).
| Key learnings |
| Results from the VENICE-1 trial suggest that venetoclax monotherapy is associated with deep and durable response rates in patients with R/R CLL, including those pretreated with BCRis. |
- Kater AP, Arslan Ö, Demirkan F, et al. Activity of venetoclax in patients with relapsed or refractory chronic lymphocytic leukaemia: analysis of the VENICE-1 multicentre, open-label, single-arm, phase 3b trial. Lancet Oncol. 2024;25(4):463-473. DOI: 1016/S1470-2045(24)00070-6

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youNewsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








