All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The Lymphoma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
An expert panel hosted by

Sequencing immune-based therapies in B-cell malignancies
with Ulric Jäger, Sagar Lonial, and Krina Patel

Saturday, June 15 | 18:00-19:30 CEST
Register nowThis independent education activity is sponsored by Bristol Myers Squibb. All content is developed independently by the faculty. Funders are allowed no direct influence on the content of this activity.
The Lymphoma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Lymphoma Hub cannot guarantee the accuracy of translated content. The Lymphoma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
FDA grants lisocabtagene maraleucel priority review for R/R CLL or SLL
By Sabina Ray
Bookmark this article
On November 9, 2023, it was announced that the U.S. Food and Drug Administration (FDA) had granted lisocabtagene maraleucel, a chimeric antigen receptor T-cell therapy, priority review for the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL). This decision is based on promising results from the TRANSCEND CLL 004 (NCT03331198) trial.1
TRANSCEND CLL 004
TRANSCEND CLL 004 is a phase I/II open-label study evaluating lisocabtagene maraleucel in patients with relapsed/refractory CLL/SLL.1 The Lymphoma Hub previously reported the promising initial results at a median follow-up of 21 months which are summarized in Figure 1.2
Figure 1. Lisocabtagene maraleucel efficacy in patients with R/R CLL/SLL*
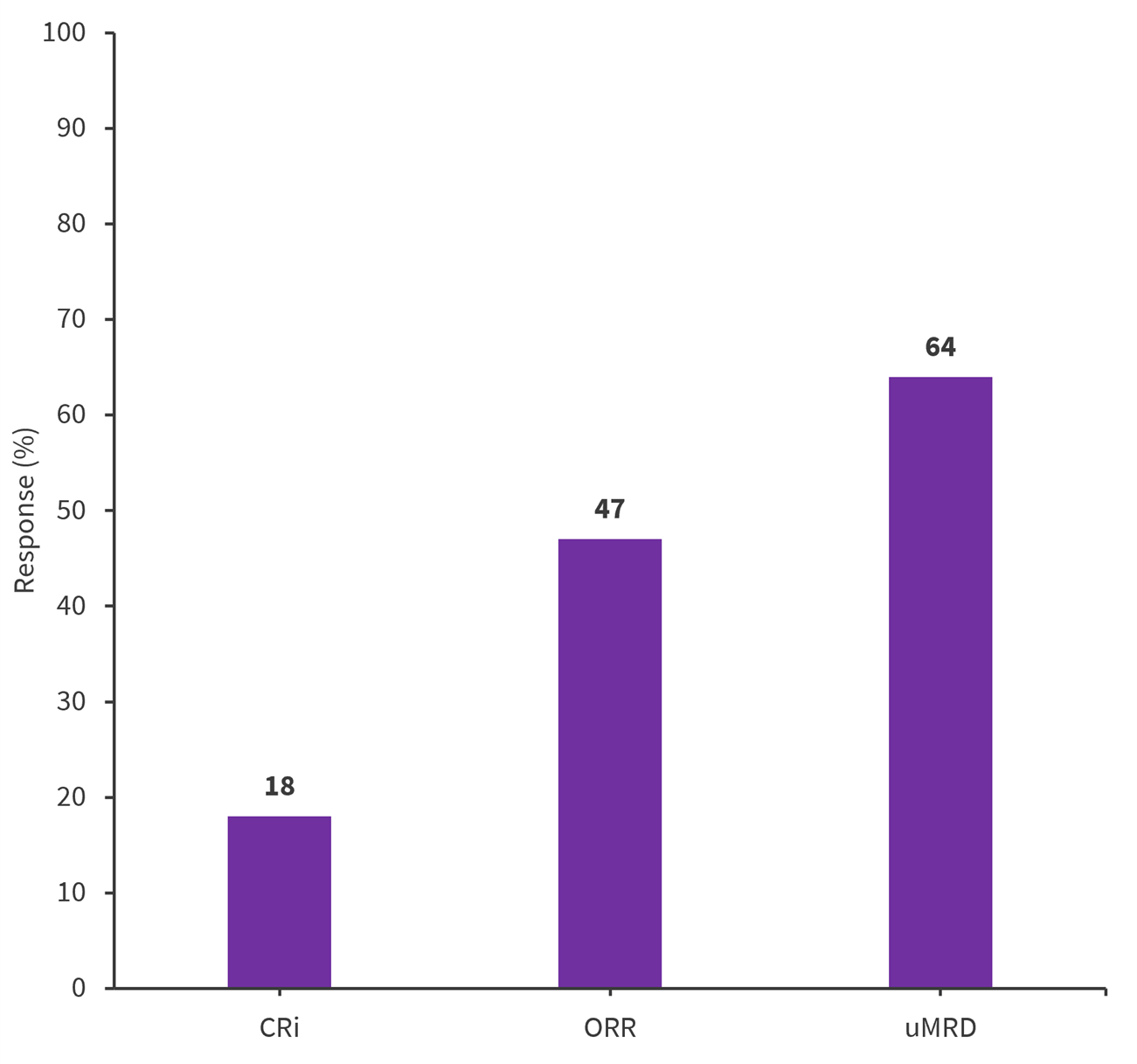
CRi, complete response with incomplete hematologic recovery; ORR, overall response rate; uMRD, undetected minimal residual disease.
*Adapted from Siddiqi, et al.2
- Business Wire. U.S. Food and Drug Administration Accepts for Priority Review Bristol Myers Squibb’s Application for Breyanzi (lisocabtagene maraleucel) for relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). https://www.businesswire.com/news/home/20231109738749/en/U.S.-Food-and-Drug-Administration-Accepts-for-Priority-Review-Bristol-Myers-Squibb%E2%80%99s-Application-for-Breyanzi-lisocabtagene-maraleucel-for-Relapsed-or-Refractory-Chronic-Lymphocytic-Leukemia-CLL-or-Small-Lymphocytic-Lymphoma-SLL. Published Nov 9, 2023. Accessed Nov 10, 2023.
- Siddiqi T, Maloney D, Kenderian S, et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1–2 study. Lancet. 2023;402(10402):641-654. DOI: 1016/S0140-6736(23)01052-8

Understanding your specialty helps us to deliver the most relevant and engaging content.
Please spare a moment to share yours.
Please select or type your specialty
 Thank you
Thank youRelated articles
Newsletter
Subscribe to get the best content related to lymphoma & CLL delivered to your inbox








